Abstract
Harvest of the rectus abdominis muscle requires an abdominal incision as well as violation of the anterior rectus sheath, creating the potential for significant surgical-site morbidity (bulges, hernias, infections, seromas). Laparoscopic or endoscopic techniques, although feasible, have not become popular among plastic surgeons due to multiple technical shortcomings. Robotic surgery on the other hand has an easier learning curve, enhanced precision, tremor elimination, motion scaling, high resolution, three-dimensional optics and an intuitive interface. As a result of these advantages, robotic surgery has permeated into the plastic surgery specialty, assuming a role in the harvest of the latissimus dorsi muscle flap and other reconstructive procedures. In this review, the authors discuss its applicability in the harvest of the rectus abdominis muscle.
Keywords: robotic surgery, rectus abdominis muscle flap, minimally invasive surgery
The rectus abdominis muscle is a workhorse flap for reconstructing challenging defects. Owing to its strategic location in the abdomen with a dual blood supply and wide arc of transposition, it can provide substantial coverage and a large volume for obliterating dead spaces while maintaining a reliable vascular supply. Using traditional approaches, however, its harvest has the potential for donor-site morbidity. We have previously highlighted the advantages of using the robot for harvesting the latissimus dorsi muscle flap.1 2 3 In this review, we discuss the applicability of this novel approach to muscle harvest for a minimally invasive harvest of the rectus abdominis muscle flap.
Main Section
Versatile Flap: Dual Blood Supply
The rectus abdominis muscle flap has two major blood supplies: the superior epigastric artery and its associated venous drainage system, which provides a cephalic arc of rotation, and the inferior epigastric artery and veins, which provide a caudal arc of rotation. It has thus found applications in perineal,4 extremity,5 chest wall,6 and even head and neck reconstruction.7 8
Complications of the Traditional Harvest Technique
Harvest of the rectus muscle is typically performed through a vertical, paramedian skin incision, followed by vertical division of the anterior rectus sheath along the length of the muscle (Fig. 1). This technique is both aesthetically undesirable and is associated with donor-site morbidity, including wound infection, seroma, abdominal bulge, and hernia. Reported bulge rates are between 1.7 and 4% with hernia rates between 0.85 and 2.9%.9 10 11 12 Overall surgical-site morbidity may be as high as 8.5 to 14.3%.9 12 Minimally invasive harvest of the rectus abdominis muscle without violation of the anterior rectus sheath is a desirable goal. This approach might not only circumvent the surgical complications associated with the traditional approach, but could also allow for minimally invasive pelvic floor reconstructions and even include the potential for intraperitoneal microvascular anastomosis.13
Fig. 1.
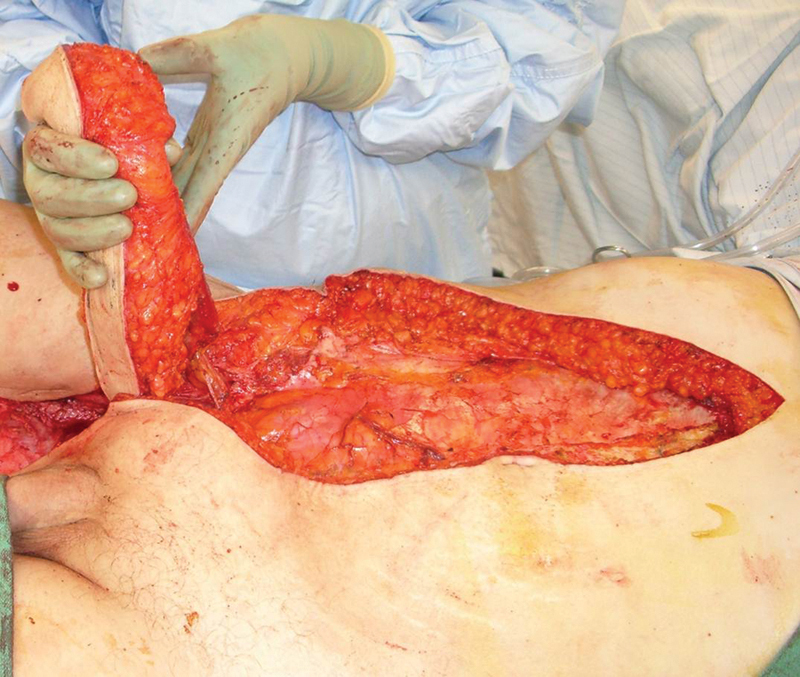
Traditional harvest of the rectus abdominis muscle requires a long paramedian incision and vertical incision of the anterior rectus sheath. This has the potential for significant donor site morbidity including seroma, pain, hernia, and bulge.
Limitations of the Endoscopic and Laparoscopic Harvest Techniques
Endoscopic extraperitoneal and traditional transperitoneal laparoscopic techniques have been attempted by some groups,14 15 16 but have not gained wide popularity among plastic surgeons. This is because the endoscopic approach still violates, to varying degrees, the integrity of anterior rectus sheath, and the laparoscopic approach requires a level of laparoscopic skills that are generally absent in modern plastic surgery training. Table 1 compares the features of both techniques with that of a robotic approach.
Table 1. Comparison of the laparoscopic, endoscopic, and robotic approaches in the harvest of the rectus abdominis muscle.
| Robotic | Endoscopy | Laparoscopy | |
|---|---|---|---|
| Ease of technique | ++ | − | − |
| Learning curve | ++ | − | − |
| Ergonomics | ++ + + | − | − |
| Instrumentation | − | + | + |
| Precision | ++ | − | − |
| Optics | ++ | − | − |
Robotic Harvest Technique
Robotic surgery has emerged during the past two decades as an innovative, minimally invasive technology.17 With enhanced precision, tremor elimination, motion scaling, high resolution, three-dimensional (3D) optics as well as an intuitive interface, it has found multiple applications across several surgical subspecialties.18 19 20 21 Additionally, for some procedures it has resulted in less blood loss, fewer transfusions, decreased postoperative pain, less risk of infection, a shorter hospital stay, less scarring, and a more rapid recovery and return to activities of daily living.22 23 24
Recently, surgeons have adopted the use of robot-assisted surgery in several reconstructive procedures, especially oropharyngeal reconstruction,25 26 27 28 motivated by a decrease in surgical-site morbidity such as lip splitting and mandibulotomy. More recently, the application of robotic-assisted muscle harvest, including the latissimus dorsi muscle, was developed by the senior author (JCS) and has been performed in several centers.1 29 Robotic harvest of the rectus abdominis muscle has now been successfully performed in at least three centers with the aim of avoiding violation of the anterior rectus sheath and hence decreasing the incidence of postoperative surgical-site complications.30 The first published case report of robotic rectus harvest was performed on a 30-year-old woman for a lower-extremity defect.13 The authors observed advantages of the robotic approach, including uninhibited 3D view of the rectus muscle and the deep inferior epigastric artery (DIEA), dexterity levels similar to human hands, and a clearer image than traditional laparoscopy. Interestingly, during the dissection, it was noted that gravity serves to autoretract the muscle and makes the perforators and inscriptions easier to visualize.
Experience with the Robotic Approach: Indications and Key Advantages
Based on our experience, robotic harvest of the rectus muscle is a viable option for plastic surgeons, and can be performed for many different types of reconstructions. Indications are classified according to the pedicle supplying the flap. A superiorly based pedicled flap is used for the coverage of anterior midline chest wall and sternal defects following oncologic resection or wound debridement. Inferiorly based flaps are used to reconstruct abdominopelvic defects where either space obliteration or visceral protection is needed: abdominoperineal resection, radical cystoprostatectomy, pelvic exenteration, and coverage of major vessels or visceral repairs. Harvest of the muscle for free-tissue transfer can also be performed robotically, and indications are primarily scalp and extremity, similar to the traditional approach.
Additionally, we have observed several key advantages associated with the robotic approach for this surgery. As outlined above, the rectus muscle is traditionally accessed through long incisions to access both its origin and insertion. With the assistance of the robot, these are reduced to ports, with less risk of wound complications and improved cosmesis. Moreover, we have seen that maintaining the integrity of the anterior rectus sheath resulted in a minimal incidence of hernias and bulges. Less tissue violation is required resulting anecdotally in markedly reduced postoperative pain and discomfort, shorter length of hospital stay, and more rapid functional recovery.
Finally, this procedure can easily be combined with other pelvic procedures. This is of great value to a complex, multidisciplinary robotic surgery program where large resections are being performed without a laparotomy. Following major, intraperitoneal robotic resections, if reconstruction is required it adds tremendous value to maintain a minimally invasive approach while providing the benefits of regional, vascularized tissue transfer. It would be disappointing for plastic surgery to be the reason for a laparotomy when so much work has gone into avoiding one. Finally, in cases of free-tissue transfer, the robot may be used for a tremor-free, technically enhanced microvascular, intracorporeal anastomosis.
Operative Procedure
Positioning and Defining Landmarks
The patient is placed in either a supine or a low lithotomy position with legs in Allen stirrups, depending on the procedure to be performed (free-tissue transfer vs. regional pelvic reconstruction). Bilateral arms are tucked at the patient's flanks after adequate padding. Care is taken to ensure proper orientation and functioning of intravenous (IV) lines. If necessary (depending on the anesthesiologist's need for access), one arm can be left abducted on the contralateral side of the rectus muscle to be harvested. The patient is well secured to the operating room table, with a strap across the chest and another one over the thighs.
Incision and Port Locations
The contralateral costal margin and iliac crest are marked along a line connecting the anterior axillary line and the anterior superior iliac spine. The midpoint between these two landmarks and 2 cm lateral to it is the desired location of the 12-mm camera port. On either side of the camera port, approximately four finger breadths away, or 1 to 2 cm from the costal margin and iliac crest, is the proposed location of the two 8-mm instrument ports (Fig. 2).
Fig. 2.
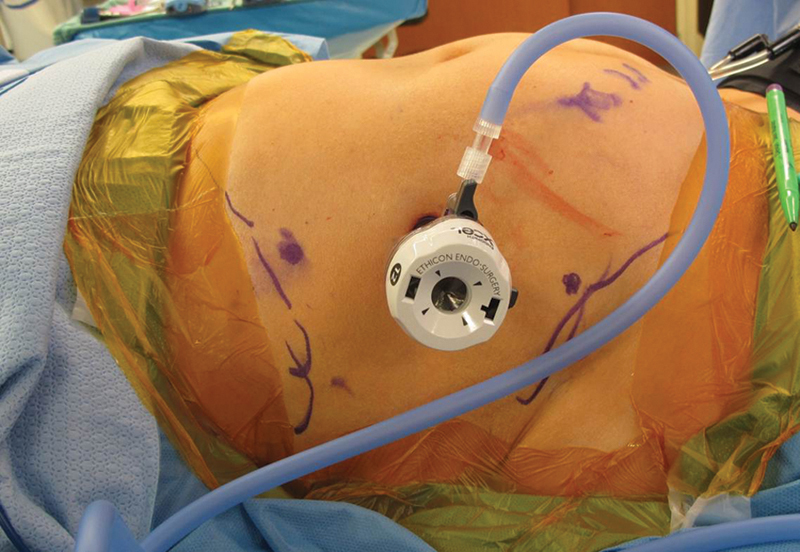
Port placement is marked along a line connecting the anterior axillary line and the anterior superior iliac spine. The midpoint between these two landmarks and 2 cm lateral to it is the desired location of the 12-mm camera port. On either side of the camera port, approximately four finger breadths away, or 1 to 2 cm from the costal margin and iliac crest, is the proposed location of the two 8-mm instrument ports.
A VARIS needle (Blitz, Voorschoten, The Netherlands) is used to access the peritoneum and attain insufflation using standard parameters (Fig. 3). Once a pneumoperitoneum of between 10 and 15 mm Hg is achieved, the port sites are verified and adjusted. The aim is to have the entire length and width of the rectus muscle (including the pedicle) within easy reach. A critical step in the harvest is to define the medial edge of the muscle, especially challenging because of the steep upward angle that must be taken from the contralateral side. The more lateral the instrument ports, the less steep this angle becomes; however, the lateral extent of the port is limited by the peritoneal reflection that marks the beginning of the retroperitoneum. This is a key consideration in port placement (Fig. 4).
Fig. 3.
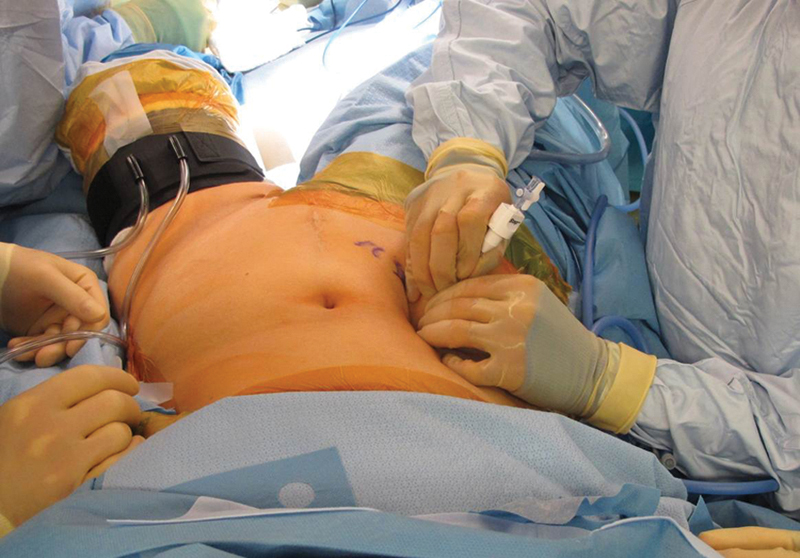
A VARIS needle (Blitz, Voorschoten, The Netherlands) is used to access the peritoneum and attain insufflation using standard parameters.
Fig. 4.
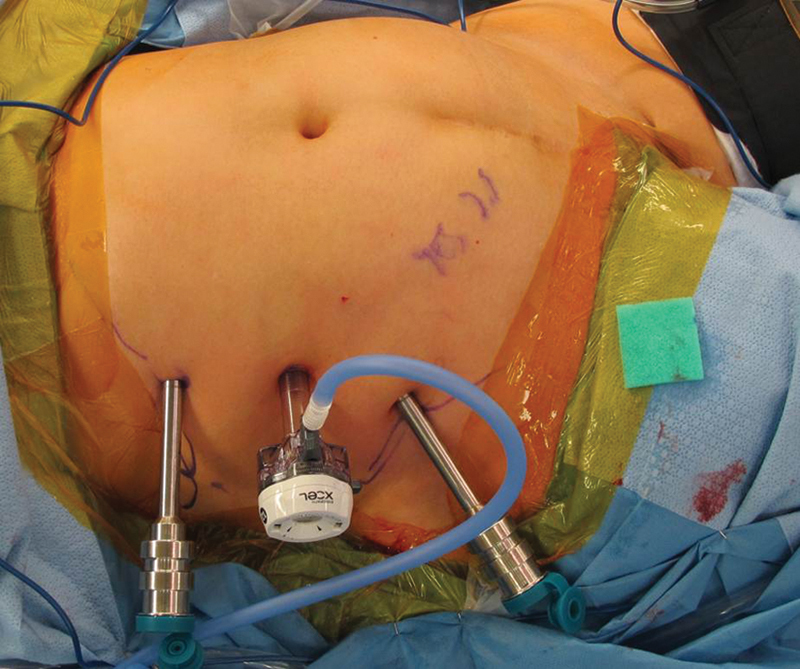
The aim of port placement is to have the entire length and width of the rectus muscle (including the pedicle) within easy reach. The more lateral the instrument ports, the less steep the angle toward the medial edge of the muscle becomes; however, the lateral extent of the port is limited by the peritoneal reflection that marks the beginning of the retroperitoneum. This is a key consideration in port placement.
Once pneumoperitoneum it attained, the camera port is placed. The scope is inserted “free hand” and the other two ports are placed under direct, laparoscopic vision.
Robotic Docking and Dissection
The surgical robot (da Vinci, Intuitive Surgical, Sunnyvale, CA) is docked on the ipsilateral side to the muscle being harvested (opposite side from the ports) and the camera arm is flexed at 90 degrees at the elbow. The camera port is then docked. Arms 1 and 2 are brought in from the sides so that the elbows are bowed out and will not conflict with the camera arm. The 8-mm ports are docked and instruments are placed (Fig. 5). A Cadiere grasper (Intuitive Surgical, Sunnyvale, CA) is placed in the nondominant arm and the monopolar scissors are placed in the dominant arm. The camera is angled to watch the instruments enter the ports and ensure that the angle of entry will not result in injury to abdominal viscera.
Fig. 5.
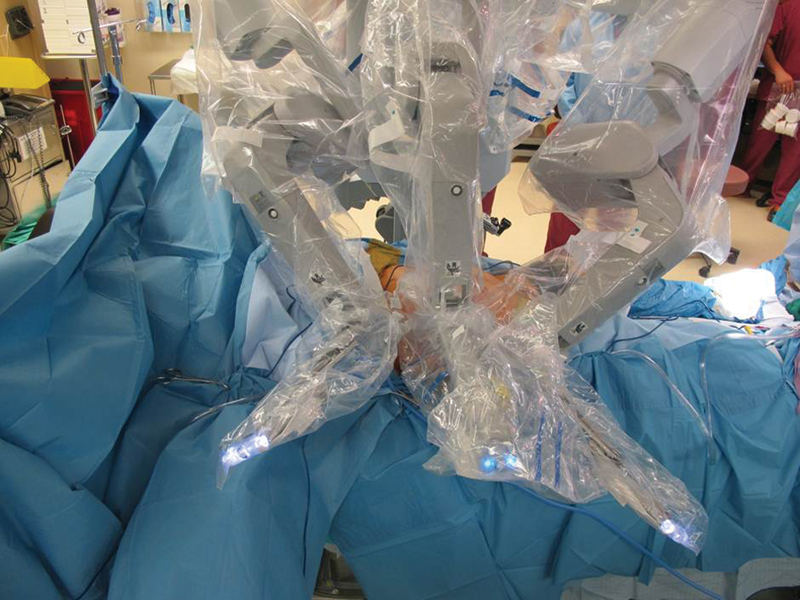
The surgical robot (da Vinci, Intuitive Surgical, Sunnyvale, CA) is docked on the ipsilateral side to the muscle being harvested (opposite side from the ports) and the camera arm is flexed at 90 degrees at the elbow. The camera port is then docked. Arms 1 and 2 are brought in from the sides so that the elbows are bowed out and will not conflict with the camera arm. The 8-mm ports are docked and instruments are placed.
Muscle Harvest
The surgeon now sits at the console and performs the robotic dissection with the first surgical assistant available at the surgical table. First, the deep inferior epigastric pedicle is identified at its branch point from the external iliac artery and vein. The peritoneum overlying the pedicle is opened sharply and the vessels are dissected from their origin to their entrance into the rectus muscle.
Posterior dissection is started by incising the posterior rectus sheath immediately lateral to the linea alba. The rectus abdominis is dissected with a combination of sharp and blunt technique in the plane between the anterior surface of the muscle and the anterior rectus sheath. This dissection continues across the entire muscle until the other side of the posterior sheath is visible on the other side of the muscle.
Two sets of structures require specific attention in this process: the perforators from the DIEA/V and the inscriptions. As in the open technique, dissection is first performed between the inscriptions, in a predominantly avascular plane. Unlike in the open procedure, however, the effect of gravity allows the muscle to be suspended by the inscriptions and facilitates their subsequent division. The DIEA/V perforators are identified as they are encountered. They can either be cauterized, bipolared, or clipped, depending on size and surgeon judgment. If clipped, a robotic clip applier can be used in place of one of the instruments; if other ports are available in the case of combined minimally invasive, pelvic procedures, a laparoscopic clip applier can be operated by the bedside assistant.
Completion of Dissection and Extraction of the Muscle
Three different scenarios for final dissection are considered based on whether the muscle is used as a pedicled flap (raised on the inferior or superior deep epigastric system) or as a free flap.
Inferiorly based rectus muscle flap (mainly used for intra-abdominal pelvic reconstruction): The inferior epigastric vessels are gently dissected down to the external iliac vessels and freed. Using the monopolar scissors, the muscle is then divided cephalad at the costal margin, and caudad between the symphysis pubis and the entrance of the pedicle into the muscle; this will completely “islandize” the muscle on the pedicle. In a controlled fashion, the muscle is then directed into the pelvis for insetting.
Superiorly based rectus muscle flap (used for midanterior chest wall-defect reconstruction): The caudal part of the muscle is divided from the symphysis pubis in a similar fashion to the inferiorly based muscle flap. The muscle is then turned over superiorly passed through an epigastric tunnel created between the chest defect and the muscle. The muscle can be partially divided, but care must be taken around the superior epigastric artery and vein.
Rectus muscle for free tissue transfer: The muscle is dissected in an identical fashion to the inferiorly based muscle flap. Once islandized, a Weck clip (Intuitive Surgical, Sunnyvale, CA) is used to divide the pedicle at its origin. The pedicle is then cut with the monopolar scissors. A 12-mm port is then placed in one of the lateral or accessory ports and an Anchor retrieval sac (Anchor Products, Addison, IL) is inserted. Once liberated, the muscle can be guided into the gallbladder bag and withdrawn from the abdomen. Closure of the 12-mm port is performed at the fascial as well as the skin level; the 8-mm ports are closed at the skin level only. An example of a donor and recipient site for the same lower extremity case is shown (Fig. 6).
Fig. 6.
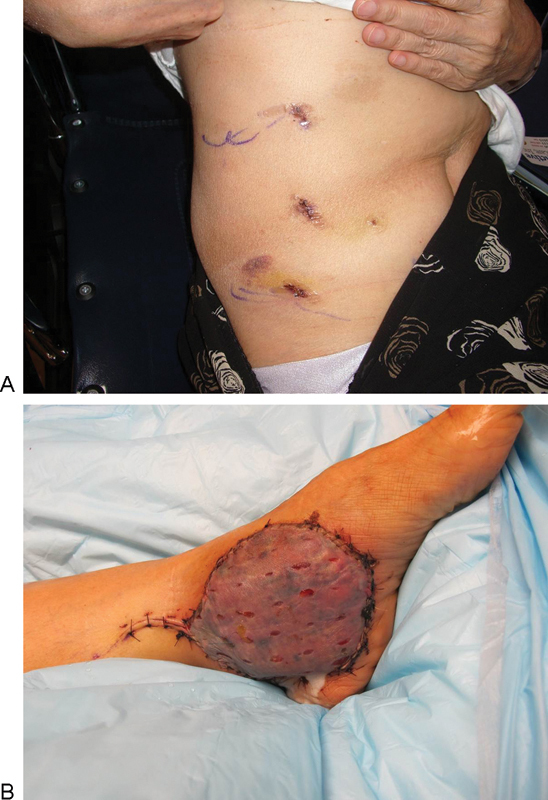
(A) The donor site is limited to the three ports because the muscle can be removed through on of the ports using a gallbladder bag. Here, a donor site is shown postoperative week 1 for an extremity case whose recipient site (B) is shown at the same postoperative visit.
The posterior sheath can be handled in a variety of ways. It can be left open with the rationale that the anterior rectus sheath is the strength layer, and leaving the posterior sheath should not increase the risk of hernia or bulge. Alternatively, the posterior sheath can be closed with a running barbed suture. Finally, a laparoscopic mesh can be used if it is felt to be necessary or beneficial. Each technique has been used to date and at this point, there is no clear evidence to support one over another.
Conclusion
Robotic surgery is a great medical innovation that is providing new horizons for minimally invasive procedures in plastic surgery. It is much more natural than traditional laparoscopic surgery, has a more favorable learning curve,31 and superior user features including enhanced precision through tremor elimination and motion scaling, high-resolution, 3D optics, as well as a highly ergonomic platform.32 The robotic rectus harvest is a novel technical innovation with many benefits that increase the versatility of an already highly useful and commonly performed flap.
References
- 1.Selber J C, Baumann D P, Holsinger F C. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg. 2012;129(6):1305–1312. doi: 10.1097/PRS.0b013e31824ecc0b. [DOI] [PubMed] [Google Scholar]
- 2.Selber J C, Baumann D P, Holsinger C F. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg. 2012;28(7):457–464. doi: 10.1055/s-0032-1315789. [DOI] [PubMed] [Google Scholar]
- 3.Selber J Breast reconstruction—current perspectives and state of the art techniques. InTech; 2013 Available at: http://www.intechopen.com/books/breast-reconstruction-current-perspectives-and-state-of-the-art-techniques/robotic-harvest-of-the-latissimus-dorsi-muscle-for-breast-reconstruction. Accessed December 10, 2013
- 4.Nigriny J F, Wu P, Butler C E. Perineal reconstruction with an extrapelvic vertical rectus abdominis myocutaneous flap. Int J Gynecol Cancer. 2010;20(9):1609–1612. doi: 10.1111/IGC.0b013e3181fc11ee. [DOI] [PubMed] [Google Scholar]
- 5.Nyame T T, Holzer P W, Helm D L, Maman D Y, Winograd J M, Cetrulo C L Jr. SPLIT rectus abdominis myocutaneous double free flap for extremity reconstruction. Microsurgery. 2014;34(1):54–57. doi: 10.1002/micr.22160. [DOI] [PubMed] [Google Scholar]
- 6.Coleman J J III, Bostwick J. Rectus abdominis muscle-musculocutaneous flap in chest-wall reconstruction. Surg Clin North Am. 1989;69(5):1007–1027. doi: 10.1016/s0039-6109(16)44935-2. [DOI] [PubMed] [Google Scholar]
- 7.Iida T, Mihara M, Yoshimatsu H. et al. Reconstruction of an extensive anterior skull base defect using a muscle-sparing rectus abdominis myocutaneous flap in a 1-year-old infant. Microsurgery. 2012;32(8):622–626. doi: 10.1002/micr.22025. [DOI] [PubMed] [Google Scholar]
- 8.Wanamaker J R, Burkey B B. Overview of the rectus abdominis myocutaneous flap in head and neck reconstruction. Facial Plast Surg. 1996;12(1):45–50. doi: 10.1055/s-2008-1064492. [DOI] [PubMed] [Google Scholar]
- 9.Ascherman J A, Seruya M, Bartsich S A. Abdominal wall morbidity following unilateral and bilateral breast reconstruction with pedicled TRAM flaps: an outcomes analysis of 117 consecutive patients. Plast Reconstr Surg. 2008;121(1):1–8. doi: 10.1097/01.prs.0000295378.43033.c4. [DOI] [PubMed] [Google Scholar]
- 10.Suominen S, Asko-Seljavaara S, von Smitten K, Ahovuo J, Sainio P, Alaranta H. Sequelae in the abdominal wall after pedicled or free TRAM flap surgery. Ann Plast Surg. 1996;36(6):629–636. doi: 10.1097/00000637-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Kroll S S, Schusterman M A, Reece G P, Miller M J, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96(3):616–619. doi: 10.1097/00006534-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Chun Y S, Sinha I, Turko A. et al. Comparison of morbidity, functional outcome, and satisfaction following bilateral TRAM versus bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2010;126(4):1133–1141. doi: 10.1097/PRS.0b013e3181ea42d3. [DOI] [PubMed] [Google Scholar]
- 13.Patel N V, Pedersen J C. Robotic harvest of the rectus abdominis muscle: a preclinical investigation and case report. J Reconstr Microsurg. 2012;28(7):477–480. doi: 10.1055/s-0031-1287674. [DOI] [PubMed] [Google Scholar]
- 14.Greensmith A, Januszkiewicz J, Poole G. Rectus abdominis muscle free flap harvest by laparoscopic sheath-sparing technique. Plast Reconstr Surg. 2000;105(4):1438–1441. doi: 10.1097/00006534-200004040-00026. [DOI] [PubMed] [Google Scholar]
- 15.Venkataramakrishnan V, Southern S J. Endoscopic rectus harvest: a simplified sheath-saving technique. Ann Plast Surg. 1997;39(6):573–577. doi: 10.1097/00000637-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bass L S Karp N S Benacquista T Kasabian A K Endoscopic harvest of the rectus abdominis free flap: balloon dissection in the fascial plane Ann Plast Surg 1995343274–279., discussion 279–280 [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim A E, Sarhane K A, Baroud J S, Atiyeh B S. Robotics in plastic surgery, a review. Eur J Plast Surg. 2012;35:571–578. [Google Scholar]
- 18.Jacobsen G, Berger R, Horgan S. The role of robotic surgery in morbid obesity. J Laparoendosc Adv Surg Tech A. 2003;13(4):279–283. doi: 10.1089/109264203322333610. [DOI] [PubMed] [Google Scholar]
- 19.Meehan J J, Elliott S, Sandler A. The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg. 2007;42(12):2110–2114. doi: 10.1016/j.jpedsurg.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Yuh B E, Hussain A, Chandrasekhar R. et al. Comparative analysis of global practice patterns in urologic robot-assisted surgery. J Endourol. 2010;24(10):1637–1644. doi: 10.1089/end.2010.0024. [DOI] [PubMed] [Google Scholar]
- 21.Zacharopoulou C, Sananes N, Baulon E, Garbin O, Wattiez A. [Robotic gynecologic surgery: state of the art. Review of the literature] J Gynecol Obstet Biol Reprod (Paris) 2010;39(6):444–452. doi: 10.1016/j.jgyn.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Chung J S, Kim W T, Ham W S. et al. Comparison of oncological results, functional outcomes, and complications for transperitoneal versus extraperitoneal robot-assisted radical prostatectomy: a single surgeon's experience. J Endourol. 2011;25(5):787–792. doi: 10.1089/end.2010.0222. [DOI] [PubMed] [Google Scholar]
- 23.Martin A D, Nunez R N, Castle E P. Robot-assisted radical cystectomy versus open radical cystectomy: a complete cost analysis. Urology. 2011;77(3):621–625. doi: 10.1016/j.urology.2010.07.502. [DOI] [PubMed] [Google Scholar]
- 24.Waters J A, Canal D F, Wiebke E A. et al. Robotic distal pancreatectomy: cost effective? Surgery. 2010;148(4):814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 25.O'Malley B W Jr, Weinstein G S, Snyder W, Hockstein N G. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116(8):1465–1472. doi: 10.1097/01.mlg.0000227184.90514.1a. [DOI] [PubMed] [Google Scholar]
- 26.Longfield E A, Holsinger F C, Selber J C. Reconstruction after robotic head and neck surgery: when and why. J Reconstr Microsurg. 2012;28(7):445–450. doi: 10.1055/s-0032-1306376. [DOI] [PubMed] [Google Scholar]
- 27.Genden E M, Desai S, Sung C-K. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009;31(3):283–289. doi: 10.1002/hed.20972. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein G S, O'Malley B W Jr, Snyder W, Hockstein N G. Transoral robotic surgery: supraglottic partial laryngectomy. Ann Otol Rhinol Laryngol. 2007;116(1):19–23. doi: 10.1177/000348940711600104. [DOI] [PubMed] [Google Scholar]
- 29.Selber J C. Robotic latissimus dorsi muscle harvest. Plast Reconstr Surg. 2011;128(2):88e–90e. doi: 10.1097/PRS.0b013e31821ef25d. [DOI] [PubMed] [Google Scholar]
- 30.Selber J, Pederson J. Paris, France: Springer; 2012. Muscle flaps; pp. 147–158. [Google Scholar]
- 31.Lim P C, Kang E, Park H. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: a case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol. 2011;120(3):413–418. doi: 10.1016/j.ygyno.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 32.Lanfranco A R, Castellanos A E, Desai J P, Meyers W C. Robotic surgery: a current perspective. Ann Surg. 2004;239(1):14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]


