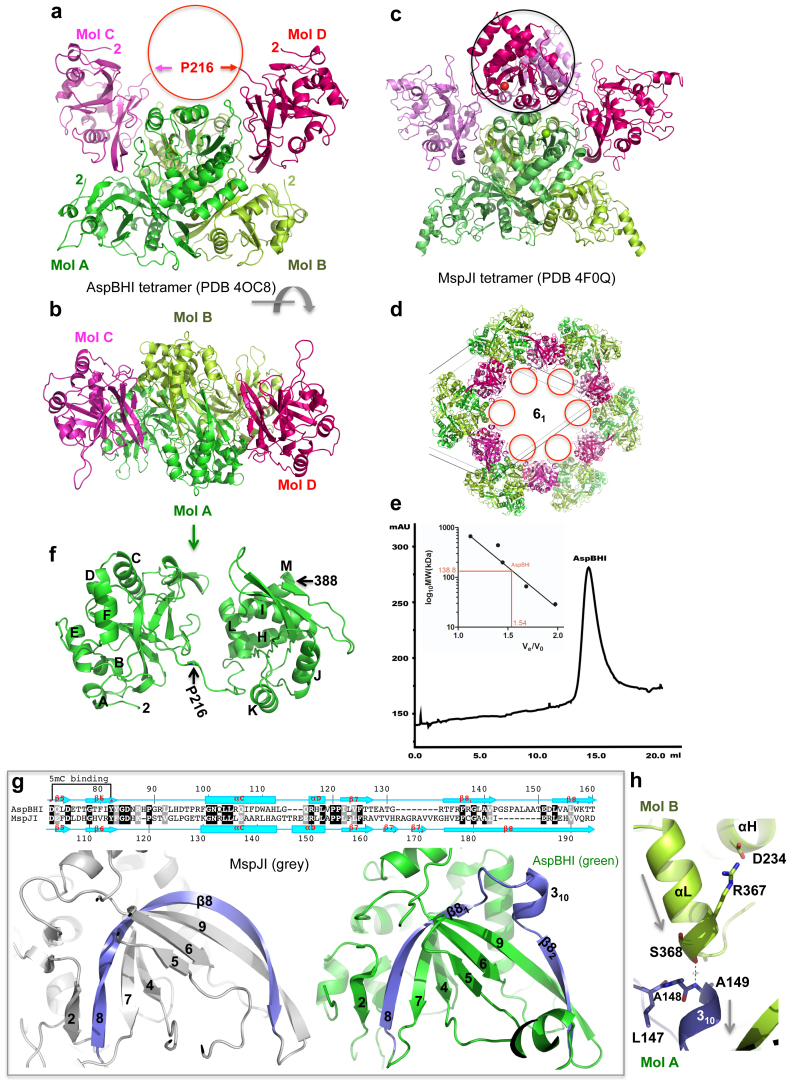
Figure 2. Structure of AspBHI.
(a) Four AspBHI monomers, A, B, C and D, form a tetramer. Molecules C and D have mobile C-terminal domains (indicated by a circle). (b) AspBHI tetramer, rotated ~90° from the view of panel (a). (c) For comparison, MspJI has an intact tetramer showing in a similar orientation of panel (a). (d) The disordered C-terminal domains of molecules C and D of AspBHI tetramer were located in the void space along the crystallographic 6-fold axis with a diameter of 100 Å. (e) Elution profile of AspBHI on Superdex 200™10/300 GL (GE Healthcare). The column buffer was 20 mM Tris-HCl (pH 7.5), 300 mM NaCl and 1 mM DTT, and 150 ng of AspBHI was loaded onto the column. The inset shows the standardization of the size exclusion column using a Gel Filtration Markers Kit for Protein Molecular Weights (SIGMA-ALDRICH, Cat. No. MWGF1000) at the time AspBHI was profiled using the same buffer. (f) Monomeric AspBHI contains two domains connected by a linker. (g) AspBHI has a discontinuity in strand β8 owing to the insertion of a 310 helix (right panel), whereas MspJI has a corresponding 20-residue-long curved strand β8 (left panel). Pairwise sequence alignment is shown above the panels. (h) The 310 helix of molecule A is involved in the dimer interface with the C-terminal helix αL of molecule B. The amino end of the 310 helix (Ala149 of molecule A) interacts with the carboxyl end of helix αL (Ser368 of molecule B). Arrows indicate helical dipoles.