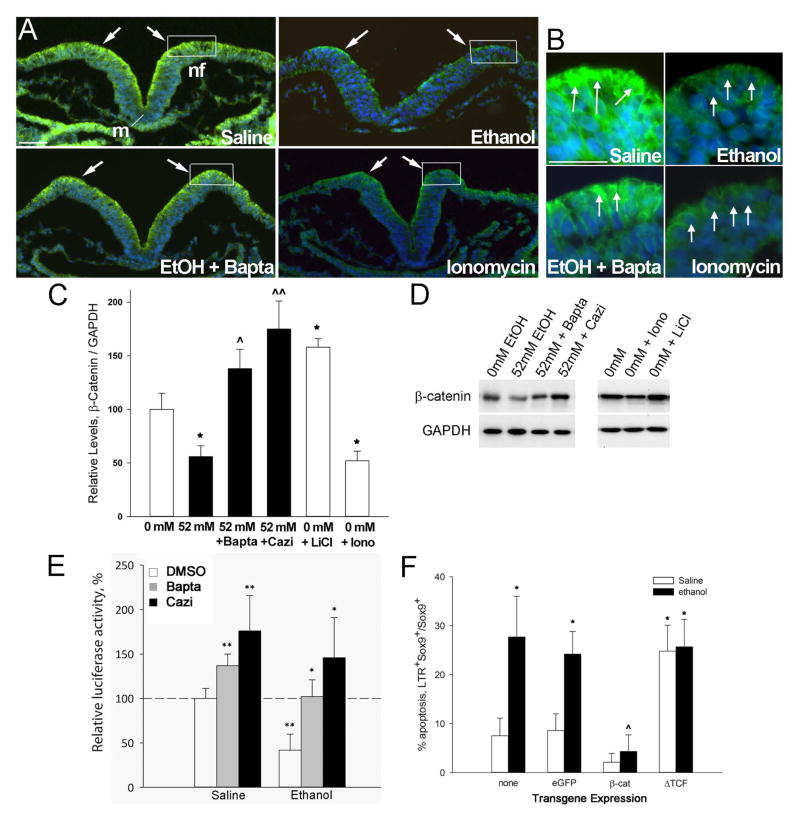
Figure 2. Ethanol depletes β-catenin from early neural progenitors.
(A, B) Calcium signals rapidly deplete β-catenin from ethanol-treated neural folds. Transverse sections through presumptive hindbrain of HH8 embryos treated as indicated for 2hr and stained for β-catenin protein (green) and nuclei (blue). Dorsal is at the top. Ethanol (52 mM) substantially reduces β-catenin content from dorsal neuroectoderm including neural crest-enriched populations (boxed region, arrows), as compared with saline-treated controls. The calcium chelator BAPTA-AM prior to ethanol exposure mitigates the β-catenin losses, whereas the calcium ionophore ionomycin similarly depletes β-catenin. (C, D) Quantitation of β-catenin protein by western blot analysis. (C) β-Catenin content, normalized to GAPDH, for neuroepithelia treated as indicated. Shown is the mean ± SEM of three independent experiments as detailed in Methods. * p <0.005 vs. saline, ^ p = 0.037 and ^^ p < 0.005 vs. ethanol-only by one-way ANOVA and Holm-Sidak comparison. (D) Representative western blots with treatments indicated, each lane evaluating a single crania. (E) Endogenous transcriptional activity of β-catenin in ethanol-treated hindbrain, measured using the pBARL luciferase reporter relative to Renilla luciferase, and normalized to the activity in saline controls. Mean ± SEM of four experiments having 6–8 embryos per treatment. * p<0.05, ** p<0.003 by ANOVA and Holm-Sidak comparison. (F) Quantitation of neural crest (Sox9+) apoptosis in r4 of ethanol-treated hindbrains transfected with β-catenin, dominant-negative TCF (ΔTCF) or eGFP control vector; “none” is non-transfected hindbrain. Mean ± SD of 3–4 embryos per treatment. * p < 0.05 vs. Saline-only, ^ p < 0.05 vs. Ethanol-only, by Kruskal-Wallis statistic. Cazi, calmidizolium.