Figure 5. Differential stress resistance and sensitization in aging, disease prevention and cancer treatment.
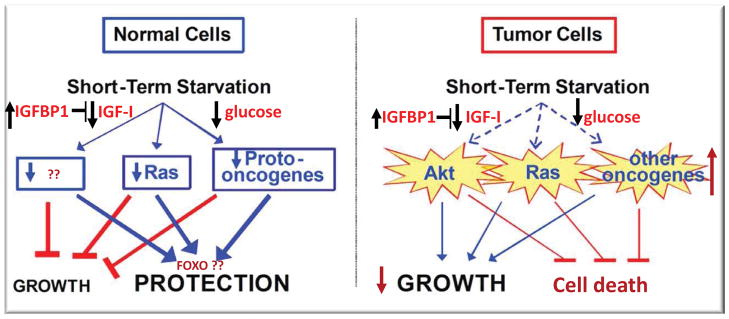
A) In both mice and humans, fasting for 2 or 5 days, respectively causes an over 60% decrease in IGF-I, a 30% or more decrease in glucose and a 5–10 fold increase in the IGF-1binding protein and inhibitor IGFBP1 (Cahill, 2006; Lee et al., 2012; Raffaghello et al., 2008; Thissen et al., 1994a; Thissen et al., 1994b). These and other endocrinological alterations affect the expression of hundreds of genes in many cell types and the consequent reduction or halting of growth and elevation in stress resistance, which may be dependent in part on FOXO and other stress resistance transcription factors. These periodically extreme conditions can promote changes, which are long-lasting and delay aging and disease independently of calorie restriction, although the cellular mechanisms responsible for these effects remain poorly understood. In the presence of chemotherapy drugs, fasting can promote the protection of normal but not cancer cells (differential stress resistance, DSR) since oncogenic pathways play central roles in inhibiting stress resistance and therefore cancer cells are unable to switch to the stress response mode; B) The extreme changes caused by fasting, and particularly the very low IGF-1and glucose levels and high IGFBP1 also generate a tumor prevention environment which promotes cancer cell death since transformed cells have acquired a number of mutations which progressively decrease their ability to adapt to extreme environments (differential stress sensitization, DSS) (Guevara-Aguirre et al., 2011; Lee et al., 2012; Lee et al., 2010).
