Abstract
Background
Hodgkin Lymphoma (HL) is uncommon in the U.S. general population; however, HL risk is elevated in people with human immunodeficiency virus (HIV) infection. Thus, despite the low HIV prevalence in the U.S, the HIV epidemic may have contributed substantially to the general population burden of HL.
Methods
We used data from 14 U.S. cancer registries in the Surveillance, Epidemiology and End Results Program that recorded HIV status of HL cases at diagnosis during 2000–2010. We computed the HIV prevalence in HL cases by demographic and tumor characteristics, the proportion of deaths among HL cases due to HIV, and 5-year mortality by HIV status.
Results
Of 22,355 HL cases, 848 (3.79%) were HIV-infected at diagnosis. HIV prevalence in HL cases was greater among males than females (6.0 vs. 1.2%). Among males, HIV prevalence was greatest among 40–59 year-olds (14.2%), non-Hispanic blacks (16.9%), Hispanics (9.9%) and among cases of lymphocyte-depleted (15.1%) and mixed cellularity HL (10.5%). Eight percent of male and 1.5% of female HL cases died from HIV. Five-year mortality was two-fold higher in HIV-infected HL cases (36.9 vs. 17.5%).
Conclusions
In the U.S., a substantial proportion of lymphocyte-depleted and mixed cellularity HL cases and HL cases among non-Hispanic black, Hispanic and middle-aged men are HIV-infected. Additionally, HIV is an important cause of death among HL cases.
Impact
Clinicians should be aware of the high prevalence of HIV in certain subgroups of HL patients and routine HIV testing should be recommended for all patients presenting with HL.
Keywords: Hodgkin lymphoma, HIV, epidemiology, SEER
Introduction
HIV is associated with the elevated risk of a number of cancers, including Hodgkin lymphoma (HL) (1). HIV increases HL risk by causing progressive immune suppression (i.e., AIDS) and likely loss of immunologic control of Epstein-Barr virus (EBV). Ninety percent of HL tumors in HIV-infected people are EBV positive, compared to 32% in HIV-uninfected people (2–4). The association with immune suppression is weaker than that observed for non-Hodgkin lymphoma (NHL). NHL rates declined dramatically with the introduction of highly active antiretroviral therapy (HAART) to treat HIV. In contrast, it is unclear whether rates of HL among people with HIV have changed in the HAART era (5–8).
The magnitude of the elevated risk of HL in people with AIDS varies across histologic subtypes of HL, with 18-fold increased risk for mixed cellularity HL, 35-fold for lymphocyte-depleted HL, 5-fold for nodular sclerosis HL, and 32-fold for unspecified HL (9, 10). Mixed cellularity is the most common HL subtype among HIV-infected individuals in contrast with the predominance of nodular sclerosis in the general population (5).
HL is uncommon in the U.S. general population, with only 9,060 cases estimated to have occurred in 2012 (11), but it is the fifth most common type of cancer in people with HIV. Despite the documented elevations in risk of HL among HIV-infected individuals, the impact of HIV-infected HL cases on the general population burden of HL has not been assessed. Using data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program for 14 U.S. regions, we estimated the proportion of HL cases during 2000–2010 who had HIV infection. We also assessed the proportion of deaths among HL cases due to HIV, and mortality among HL cases according to HIV status.
Materials and Methods
Data Sources
Data on incident HL cases were derived from 14 U.S. SEER population-based cancer registries for 2000–2010. These registries (Connecticut, Hawaii, New Mexico, Utah, Atlanta, Detroit, Seattle-Puget Sound, Los Angeles, San Francisco-Oakland, San Jose-Monterey, greater California, New Jersey, Louisiana, Kentucky) represent 24.7% of the U.S. population.
HL cases and histologic subtypes were defined using the SEER lymphoma subtype recode based on classifications proposed by the International Lymphoma Epidemiology Consortium (InterLymph) Pathology Working Group: nodular lymphocyte predominant, lymphocyte-rich, mixed cellularity, lymphocyte-depleted, nodular sclerosis, and classical Hodgkin lymphoma, not otherwise specified (NOS) (12). Disease staging was based on the Ann Arbor classification system, according to the extent of tumor and the presence or absence of “B” symptoms (i.e., systemic symptoms of night sweats, fever and weight loss) (13).
SEER registries recorded HIV serostatus at the time of cancer diagnosis (i.e., “HIV flag”) as part of the extent of disease field for individuals diagnosed with HL (14, 15). However, 61% of HL cases had unknown values for the HIV flag. We classified these cases as HIV-uninfected, as the majority of cases with known values were HIV-uninfected. For HL cases who died, SEER records the underlying cause of death from death certificates. In a sensitivity analysis, we re-classified HL cases without a positive HIV flag, but with HIV listed as the cause of death, as HIV-infected. The number of reclassified cases was small (n=43, or 0.20% of cases without a positive HIV flag), supporting our assignment of HL cases with an unknown HIV flag to uninfected status. For comparison, 24.5% of deaths among HL cases classified as HIV-infected by the HIV flag had HIV as the cause of death.
Statistical Analysis
We computed the prevalence of HIV in HL cases by sex, age group, race/ethnicity, HIV prevalence of the registry catchment area, histology, stage and the presence of “B” symptoms. Registry catchment areas were classified as having high (San Francisco-Oakland, New Jersey, Louisiana, Atlanta, Los Angeles, Detroit, Connecticut) or low HIV prevalence (Seattle-Puget Sound, greater California, Hawaii, San Jose-Monterey, Kentucky, New Mexico, Utah) compared to the national average (0.28% HIV prevalence) based on Centers for Disease Control and Prevention data from 2011 (16).
For HL cases who died, the underlying cause of death was ascertained by SEER using death certificates and was classified as due to HIV (ICD-10: B20-B24), HL (ICD-10: C81) or other causes (17). We computed the fraction of deaths among HL cases who had HIV as the underlying cause of death. The cumulative 5-year overall mortality was estimated for HIV-infected and HIV-uninfected cases of HL with the Kaplan-Meier method. The cumulative 5-year mortality due to HL and HIV (only for HIV-infected cases) was also estimated, treating other causes of death as competing events, using the survival session module in SEER*Stat (18).
Results
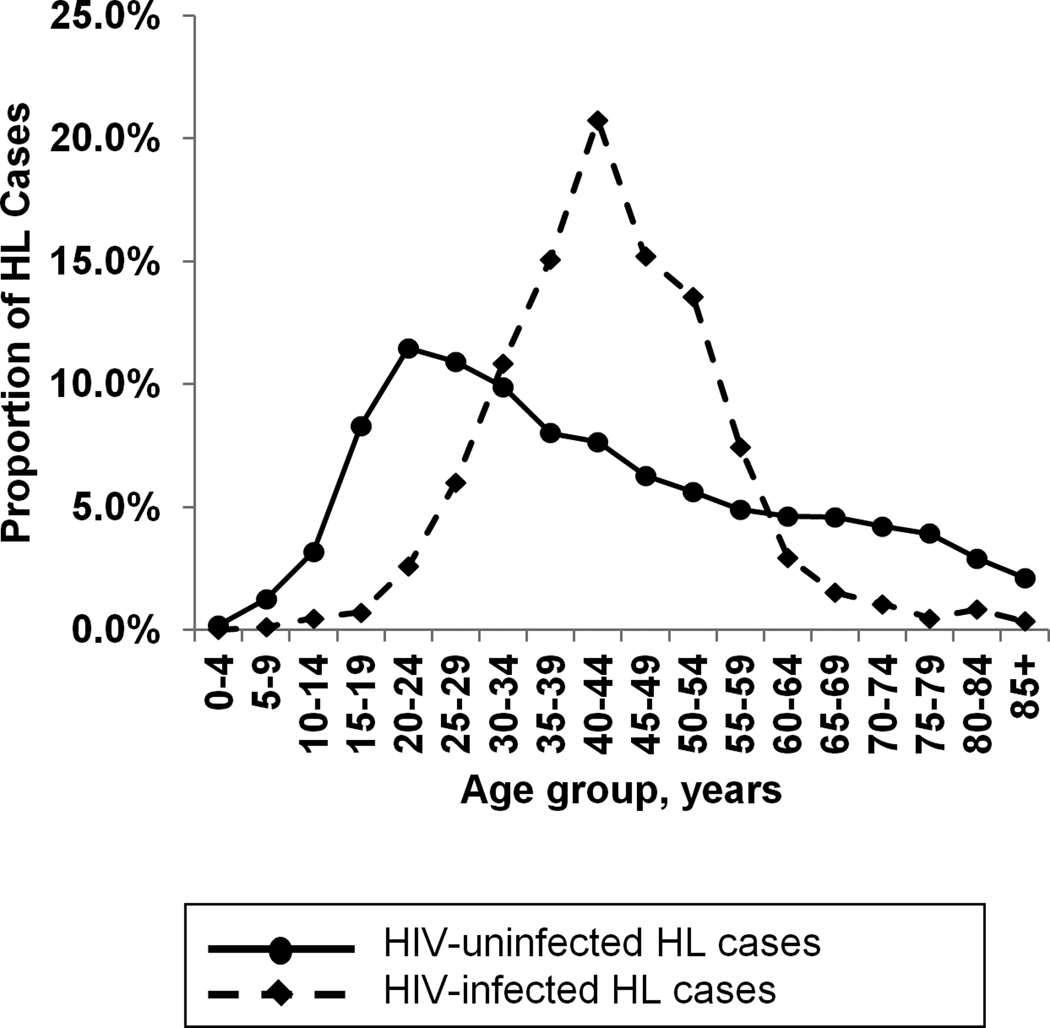
During 2000–2010, a total of 22,355 HL cases were diagnosed in 14 US SEER regions. Of these cases, 848 (3.8%) were HIV-infected at the time of diagnosis. Table 1 presents the characteristics of HL cases by HIV status. Compared to HLs in people without HIV infection, a larger proportion of HLs in people with HIV infection were mixed cellularity (25.0% vs. 12.2%), and a smaller proportion were nodular sclerosis (30.7% vs. 59.6%). HIV-infected cases were predominantly male (86.2%), while HIV-uninfected cases were more evenly divided between genders (53.7% male). The early peak in HL diagnoses in HIV-uninfected cases among 20–29 year-olds was not observed in HIV-infected cases; instead, 82.9% of HIV-infected cases occurred in 30–59 year-olds (Figure 1). The median age at Hodgkin lymphoma diagnosis was between 40 and 44 years for HIV-infected cases and between 35 and 39 years for HIV-uninfected cases. HIV-infected cases were more likely to be diagnosed at advanced stages (stage IV: 41.5% of HIV-infected vs. 17.0% of HIV-uninfected individuals) and with “B” symptoms (57.4% of HIV-infected versus 34.2% of HIV-uninfected cases).
Table 1.
Characteristics of HIV-uninfected and HIV-infected Hodgkin lymphoma cases in 14 U.S. SEER registries, 2000–2010
| HIV-uninfected | HIV-infected | |
|---|---|---|
| N (%) | N (%) | |
| Total | 21,507 (100) | 848 (100) |
| Sex | ||
| Male | 11,539 (53.7) | 731 (86.2) |
| Female | 9,968 (46.4) | 117 (13.8) |
| Age group | ||
| 0–9 | 314 (1.5) | 1 (0.1) |
| 10–19 | 2,369 (11.5) | 10 (1.2) |
| 20–29 | 4,814 (22.4) | 73 (8.6) |
| 30–39 | 3,848 (17.9) | 220 (25.9) |
| 40–49 | 2,994 (13.9) | 305 (36.0) |
| 50–59 | 2,262 (10.5) | 178 (21.0) |
| 60–69 | 1,983 (9.2) | 38 (4.5) |
| 70+ | 2,823 (13.1) | 23 (2.7) |
| Race/ethnicity | ||
| Non-Hispanic white | 14,778 (68.7) | 343 (40.5) |
| Non-Hispanic black | 2,105 (9.8) | 225 (30.1) |
| Hispanic | 3,347 (15.6) | 229 (27.0) |
| Other | 1,277 (5.9) | 21 (2.5) |
| Registry HIV prevalence | ||
| Below national average | 9,917 (46.1) | 264 (31.1) |
| Above national average | 11,590 (53.9) | 584 (68.9) |
| Histological subtype | ||
| Lymphocyte-rich | 679 (3.2) | 16 (1.9) |
| Mixed cellularity | 2,632 (12.2) | 212 (25.0) |
| Lymphocyte-depleted | 268 (1.3) | 31 (3.7) |
| Nodular sclerosis | 12,819 (59.6) | 260 (30.7) |
| Classical NOS | 4,160 (19.3) | 321 (37.9) |
| Nodular lymphocyte predominant | 949 (4.4) | 8 (0.9) |
| Ann Arbor Stage | ||
| Stage I | 3,956 (18.4) | 121 (14.3) |
| Stage II | 8,489 (39.5) | 149 (17.6) |
| Stage III | 4,115 (19.1) | 190 (22.4) |
| Stage IV | 3,660 (17.0) | 352 (41.5) |
| Unknown | 1,287 (6.0) | 36 (4.3) |
| “B” Symptoms | ||
| Absent | 9,445 (43.9) | 245 (28.9) |
| Present | 7,356 (34.2) | 487 (57.4) |
| Unknown | 4,706 (21.9) | 116 (13.7) |
Figure 1.
Proportion of Hodgkin lymphoma cases in each 5-year age group in 14 SEER registries during 2000–2010, by HIV status. The solid line indicates HIV-uninfected HL cases and the dashed line indicates HIV-infected cases.
Table 2 displays the prevalence of HIV infection among subgroups of HL cases. HIV prevalence was greater among male (5.96%) than among female (1.16%) HL cases. Among male HL cases, HIV prevalence was highest (14.2%) in 40–49 year-olds. Non-Hispanic black and Hispanic males had the highest proportion of HL cases with HIV (16.9% and 9.89%, respectively). The fraction of male HL cases with HIV infection was higher in regions with HIV prevalence above the national average than in regions with HIV prevalence below the national average (7.65 vs. 3.99%). Among males, a higher proportion of lymphocyte-depleted HL (15.1%), mixed cellularity HL (10.5%) and classical HL NOS (10.8%) were HIV-infected compared to lymphocyte-rich (3.42%), nodular sclerosis (3.22%) and nodular lymphocyte predominant HL (0.60%). Also, among males, HIV prevalence was higher in stage IV HL cases and HL cases with “B” symptoms (13.0% and 9.13%, respectively) compared to less advanced cases. Patterns were similar among females, albeit with much lower prevalence (Table 2).
Table 2.
Prevalence of HIV among cases of Hodgkin lymphoma in 14 SEER registries, 2000–2010.
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| N | Proportion with HIV, % |
95% CIs | N | Proportion with HIV, % |
95% CIs | |
| Total | 12,270 | 5.96 | (5.54–6.37) | 10,085 | 1.16 | (0.95–1.37) |
| Age group | ||||||
| 0–9 | 227 | 0.44 | 0–1.30 | 88 | 0 | (0–0) |
| 10–19 | 1,267 | 0.55 | 0.14–0.96 | 1,212 | 0.25 | (0–0.53) |
| 20–29 | 2,448 | 2.04 | 1.48–2.60 | 2,439 | 0.94 | (0.56–1.33) |
| 30–39 | 2,199 | 8.87 | 7.68–10.1 | 1,869 | 1.33 | (0.82–1.88) |
| 40–49 | 1,982 | 14.2 | 12.6–15.7 | 1,317 | 1.82 | (1.10–2.54) |
| 50–59 | 1,518 | 10.1 | 8.63–11.7 | 922 | 2.60 | (1.58–3.63) |
| 60–69 | 1,182 | 2.54 | 1.64–3.43 | 839 | 0.95 | (0.30–1.61) |
| 70+ | 1,447 | 0.90 | 0.41–1.39 | 1,399 | 0.71 | (0.27–1.16) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 8,243 | 3.64 | (3.24–4.04) | 6,878 | 0.63 | (0.44–0.81) |
| Non-Hispanic black | 1,273 | 16.9 | (14.8–18.9) | 1,087 | 3.68 | (2.56–4.80) |
| Hispanic | 2,032 | 9.89 | (8.59–11.2) | 1,544 | 1.81 | (1.15–2.48) |
| Other | 707 | 2.08 | (1.04–3.12) | 576 | 1.04 | (0.21–1.87) |
| Registry HIV prevalence | ||||||
| Below national average | 5,669 | 3.99 | (3.48–4.50) | 4,096 | 0.78 | (0.51–1.05) |
| Above national average | 6,601 | 7.65 | (7.01–8.29) | 5,074 | 1.32 | (1.01–1.63) |
| Histological subtype | ||||||
| Lymphocyte-rich | 423 | 3.42 | (1.72–5.13) | 257 | 0.39 | (0–1.15) |
| Mixed cellularity | 1,796 | 10.5 | (9.05–11.9) | 1,048 | 2.29 | (1.38–3.20) |
| Lymphocyte-depleted | 185 | 15.1 | (9.97–20.3) | 114 | 2.63 | (0–5.57) |
| Nodular sclerosis | 6,561 | 3.22 | (2.79–3.64) | 6,518 | 0.75 | (0.54–0.96) |
| Classical NOS | 2,628 | 10.8 | (9.66–12.0) | 1,853 | 1.94 | (1.31–2.57) |
| Nodular lymphocyte predominant | 662 | 0.60 | (0.01–1.19) | 295 | 1.36 | (0.04–2.68) |
| Ann Arbor Stage | ||||||
| Stage I | 2,354 | 4.33 | (3.51–5.16) | 1,600 | 1.19 | (0.66–1.72) |
| Stage II | 4,148 | 2.87 | (2.36–3.38) | 4,071 | 0.64 | (0.39–0.88) |
| Stage III | 2,567 | 6.19 | (5.26–7.13) | 1,523 | 1.61 | (0.99–2.24) |
| Stage IV | 2,493 | 13.0 | (11.6–14.3) | 1,360 | 1.59 | (0.93–2.25) |
| Unknown | 680 | 3.95 | (2.52–5.39) | 562 | 1.23 | (0.32–2.14) |
| “B” Symptoms | ||||||
| Absent | 4,966 | 3.99 | (3.44–4.53) | 4,724 | 0.99 | (0.71–1.28) |
| Present | 4,731 | 9.13 | (8.31–9.95) | 3,112 | 1.77 | (1.30–2.23) |
| Unknown | 2,472 | 3.93 | (3.18–4.68) | 2,249 | 0.67 | (0.33–1.00) |
In a sensitivity analysis, we re-classified 43 HL cases without a positive HIV flag, but with HIV as the cause of death, as HIV-infected. The overall proportions of HL cases with HIV infection were similar (6.22% in men, 1.26% in women), and the patterns were consistent with our main analysis (not shown).
Overall, 8.33% of deaths in male HL cases and 1.50% of deaths in female HL cases were due to HIV (Table 3). The patterns for the fraction of deaths due to HIV infection were similar to those described for HIV prevalence in HL cases. Among males, the proportion of deaths among HL cases that were due to HIV infection was particularly high among 30–39 and 40–49 year-olds (21.0% and 24.6%, respectively), and non-Hispanic blacks and Hispanics (24.7% and 12.9%, respectively). In addition, a large fraction of deaths among male cases of mixed cellularity (9.64%) and lymphocyte-depleted (15.4%) HLs, stage IV HLs (14.7%) and HLs with “B” symptoms (11.3%) were due to HIV.
Table 3.
Proportion of deaths in Hodgkin lymphoma cases with HIV as the cause of death in 14 SEER registries, 2000–2010.
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| N | HIV deaths, % |
95% CIs | N | HIV deaths, % |
95% CIs | |
| Total | 2,689 | 8.33 | (7.29–9.37) | 1799 | 1.50 | (0.94–2.06) |
| Age group | ||||||
| 0–9 | 9 | 0 | (0–0) | 2 | 0 | (0–0) |
| 10–19 | 49 | 0 | (0–0) | 68 | 0 | (0–0) |
| 20–29 | 187 | 3.21 | (0.81–6.74) | 131 | 0 | (0–0) |
| 30–39 | 252 | 21.0 | (16.5–27.3) | 107 | 5.61 | (1.25–9.97) |
| 40–49 | 358 | 24.6 | (21.3–30.9) | 139 | 10.1 | (5.07–15.1) |
| 50–59 | 422 | 14.2 | (10.1–17.2) | 177 | 2.82 | (0.38–5.27) |
| 60–69 | 463 | 3.02 | (1.29–4.53) | 279 | 0.36 | (0–1.06) |
| 70+ | 949 | 0.32 | (0–0.79) | 896 | 0.11 | (0–0.33) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1,560 | 4.56 | (3.59–5.53) | 1,268 | 0.47 | (0.10–0.85) |
| Non-Hispanic black | 280 | 24.7 | (20.0–29.3) | 189 | 7.94 | (4.08–11.8) |
| Hispanic | 391 | 12.9 | (9.84–16.0) | 264 | 2.27 | (0.47–4.07) |
| Other | 104 | 2.36 | (0–5.00) | 78 | 0 | (0–0) |
| Registry HIV prevalence | ||||||
| Below national average | 1,212 | 3.88 | (2.79–4.96) | 807 | 0.87 | (0.23–1.51) |
| Above national average | 1,477 | 12.0 | (10.3–13.6) | 992 | 2.02 | (1.14–2.89) |
| Histological subtype | ||||||
| Lymphocyte-rich | 71 | 2.82 | (0–6.67) | 52 | 0 | (0–0) |
| Mixed cellularity | 477 | 9.64 | (6.99–12.3) | 332 | 2.71 | (0.96–4.46) |
| Lymphocyte-depleted | 91 | 15.4 | (7.97–22.8) | 45 | 1.92 | (0–5.66) |
| Nodular sclerosis | 1,065 | 4.60 | (3.34–5.86) | 700 | 0.63 | (0.08–1.18) |
| Classical NOS | 928 | 12.2 | (10.1–14.3) | 753 | 2.24 | (0.99–3.49) |
| Nodular lymphocyte predominant | 57 | 0 | (0–0) | 32 | 0 | (0–0) |
| Ann Arbor Stage | ||||||
| Stage I | 402 | 6.47 | (4.06–8.87) | 294 | 1.36 | (0.04–2.68) |
| Stage II | 571 | 3.50 | (1.99–5.01) | 444 | 1.58 | (0.42–2.74) |
| Stage III | 634 | 5.84 | (4.01–7.66) | 413 | 0.24 | (0–0.72) |
| Stage IV | 875 | 14.7 | (12.4–17.1) | 476 | 2.73 | (1.27–4.20) |
| Unknown | 207 | 5.80 | (2.61–8.98) | 172 | 1.16 | (0–2.76) |
| “B” Symptoms | ||||||
| Absent | 741 | 5.67 | (4.00–7.33) | 599 | 0.83 | (0.11–1.56) |
| Present | 1,234 | 11.3 | (9.50–13.0) | 692 | 2.31 | (1.19–3.43) |
| Unknown | 714 | 6.02 | (4.28–7.77) | 508 | 1.18 | (0.24–2.12) |
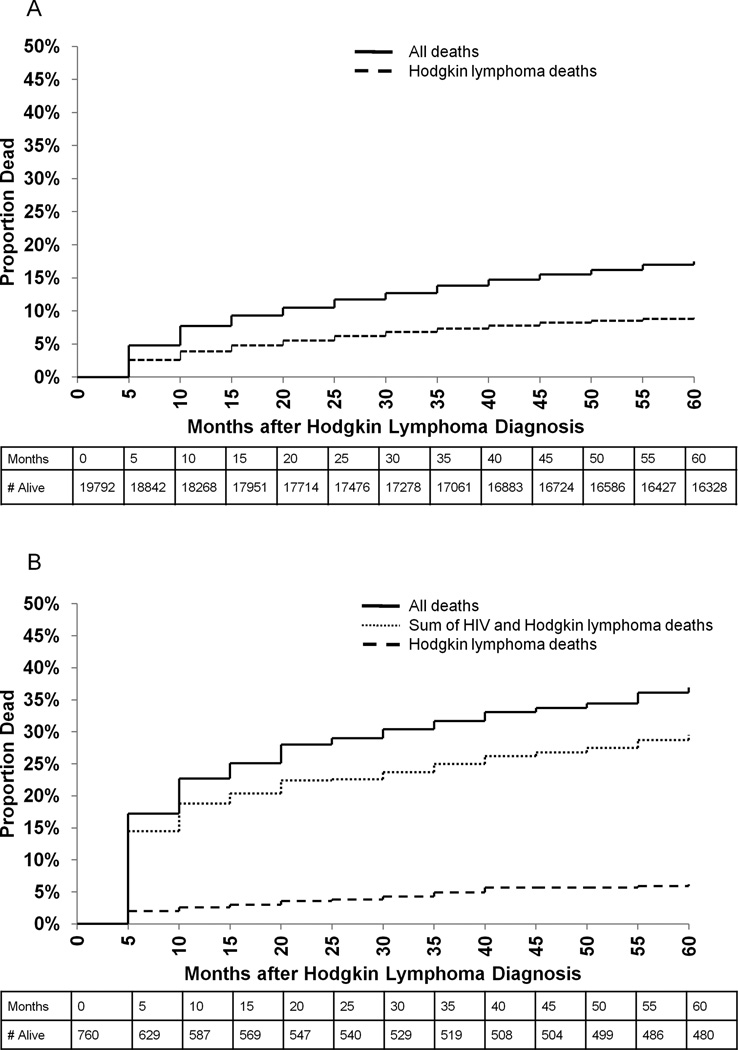
The 5-year risk of death among HIV-infected HL cases was 36.9% compared to 17.5% among HIV-uninfected HL cases (Figures 2A and 2B). The majority of deaths among HIV-infected HL cases were due to HIV (65.6% of deaths; absolute risk of death due to HIV: 23.3% in 5 years). In contrast, the risk of death due to HL was similar among HIV-uninfected and HIV-infected HL cases (9.0% vs. 6.2% in 5 years, respectively).
Figure 2.
Five-year overall and cause-specific survival of Hodgkin lymphoma cases by HIV status in 14 SEER registries during 2000–2010. Panel A includes HIV-uninfected HL cases, and panel B includes HIV-infected HL cases. In both panels, the solid line indicates overall mortality and the dashed line indicates Hodgkin lymphoma-specific mortality. In panel B, the dotted line indicates the sum of HIV and Hodgkin lymphoma-specific mortality.
Discussion
In recent decades, HIV has had an important impact on the overall general population burden of HL in the U.S., and is an important cause of mortality in HL cases. For 14 U.S. areas during 2000–2010, we estimated that 6% of HL cases in men and 1% of HL cases in women were HIV-infected. Additionally, 8% of deaths in male HL cases and 1.5% of deaths in female HL cases were due to HIV infection. At 5 years after HL diagnosis, the overall risk of death was twice as high in HIV-infected HL cases compared to HIV-uninfected HL cases.
We have shown that a substantial fraction of HL cases are HIV-infected and an even larger fraction of deaths in HL cases are due to HIV. It has long been recognized that the risk of HL is elevated in HIV-infected individuals (19). However, for clinicians, a diagnosis of HL may not raise the same level of suspicion of HIV infection as other HIV-related cancers (e.g., Kaposi sarcoma or anal cancer in younger men), and may be less likely to result in an HIV test. Therefore, clinicians need to be aware that for mixed cellularity and lymphocyte-depleted HL, and for HLs occurring among 30–59 year-old men and minorities, a notable fraction may be HIV-infected. For HIV-infected HL patients, especially those with substantial immunosuppression, clinicians may recommend initiation of HAART before or concurrently with chemotherapy (20). Undiagnosed and untreated HIV-infected HL patients who undergo chemotherapy may have an increased risk of AIDS-related or chemotherapy-related complications. Currently, treatment guidelines issued by the National Comprehensive Cancer Network encourage HIV testing for Hodgkin lymphoma patients with “risk factors for HIV or unusual disease presentations,” but do not classify HIV testing as essential (21). Given the high prevalence of HIV infection in HL patients, and the United States Preventive Services Task Force’s current recommendation for universal HIV testing in the U.S (22), we would argue that HIV testing for all patients with HL should be incorporated into cancer management guidelines.
Among males, the fraction of HL cases with HIV is quite high in specific demographic subgroups. For example, 1 in 7 HL cases in 40–49 year-old males, 1 in 6 HL cases in non-Hispanic black males and 1 in 10 HL cases in Hispanic males were HIV-infected. Additionally, a quarter of all deaths occurring among HL cases in 40–49 year-old and non-Hispanic black men were due to HIV. These demographic patterns are likely driven by the high prevalence of HIV in these subgroups of the general population (16). In addition, the incidence rate of HL in the general population in middle age groups is lower than in younger and older age groups; thus, the contribution of HIV-infected cases has a greater impact among middle-aged men.
The fraction of HL cases with HIV also varied by tumor histology, stage and the presence of “B” symptoms. Among males, 1 in 10 cases of mixed cellularity HL and 1 in 7 cases of lymphocyte-depleted HL were HIV-infected. Further, 1 in 8 stage IV HL cases and 1 in 11 HL cases with “B” symptoms were HIV-infected. Similar proportions of death due to HIV were observed in these subgroups. These results are consistent with many prior studies that have shown mixed cellularity and lymphocyte-depleted HL to be more common among HIV-infected individuals, and have shown the risk of these subtypes is particularly high (5, 9, 23, 24). These subtypes are also known to have the strongest association with EBV (25). EBV is present in the majority of tumors in HIV-infected cases, with one prior study reporting 90% of HIV-infected cases as EBV positive (4). HIV-related immune suppression likely increases the risk of HL by impairing control of EBV infection (4). The role of immune suppression in increasing the risk of HL is further supported by the 4-fold increased risk of HL observed among solid organ transplant recipients (26).
The 5-year mortality was two-fold higher in HIV-infected HL cases compared to HIV-uninfected HL cases. These results contrast with findings in recent trials, which have shown that overall 5-year survival following standard treatment for HL is the same for HIV-infected and HIV-uninfected individuals (27, 28). In our study, poorer survival in HIV-infected HL cases was largely driven by deaths due to HIV, which may reflect less adequate treatment of HIV outside a clinical trial setting. In contrast to overall survival, the 5-year risk of dying from HL was similar among HIV-infected individuals. This result may seem counterintuitive, given the large fraction of HIV-infected HL cases that are late stage and have systemic symptoms at diagnosis. However, the high risk of death from HIV (as a competing event) may preclude death due to HL. Additionally, it is possible that the underlying cause of death in an HIV-infected individual is preferentially coded as HIV, even if the death was ultimately due to another cause, leading to a smaller number of deaths coded as HL.
The primary limitation in this study was incomplete ascertainment of the HIV status of HIV-infected cases with the SEER HIV flag. Prior studies have shown the SEER flag to be >90% sensitivity in the identification of HIV-infected NHL (14, 15); however, similar analyses have not been carried out for HL. In a sensitivity analysis, the reclassification of a small number of HL cases without a positive HIV flag, but with HIV as a cause of death, had little impact on our estimates. Because we classified all individuals with an unknown HIV flag as HIV-uninfected, our estimates are conservative. We also note that the 14 SEER registries included in our analysis may not be representative of the entire U.S. population. In particular, they included registries with wide variation in HIV prevalence in the general population (e.g., San Francisco-Oakland: 0.55% and Utah: 0.11%) (16). However, because the proportion of HL cases with HIV varies based on regional HIV prevalence, the proportion of HL cases with HIV in the entire U.S. probably lies somewhere between our estimates for low and high prevalence regions and therefore close to our overall estimate.
In conclusion, we have presented the first estimates of the contribution of HIV-infected cases to the overall HL burden in 14 regions of the U.S. across subgroups defined by demographics and tumor characteristics. A substantial fraction of HL cases occurring in middle-aged, non-Hispanic black and Hispanic men are HIV-infected, as well as a large fraction of mixed cellularity and lymphocyte-depleted HL cases. As the HIV-infected population in the U.S. continues to grow, the absolute number of HIV-infected HL cases will likely rise, resulting in an increasing proportion of HL cases in the general population with HIV infection (29). Clinicians should be aware of the high prevalence of HIV in certain subgroups of HL patients, particularly middle-aged males and African-American males, and routine HIV testing should be recommended for all patients presenting with HL.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute.
Footnotes
Presented as a poster at the 46th Annual Society for Epidemiologic Research Meeting, Boston, MA, June 18, 2013.
Reference List
- 1.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohlius J, Schmidlin K, Boue F, Fatkenheuer G, May M, Caro-Murillo AM, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4(+) T-cell lymphocytes. Blood. 2011;117:6100–6108. doi: 10.1182/blood-2010-08-301531. [DOI] [PubMed] [Google Scholar]
- 3.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 4.Glaser SL, Clarke CA, Gulley ML, Craig FE, DiGiuseppe JA, Dorfman RF, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 5.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford GM, Rickenbach M, Lise M, Dal ML, Battegay M, Bohlius J, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113:5737–5742. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 7.Bohlius J, Schmidlin K, Boue F, Fatkenheuer G, May M, Caro-Murillo AM, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4(+) T-cell lymphocytes. Blood. 2011;117:6100–6108. doi: 10.1182/blood-2010-08-301531. [DOI] [PubMed] [Google Scholar]
- 8.Lanoy E, Rosenberg PS, Fily F, Lascaux AS, Martinez V, Partisani M, et al. HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood. 2011;118:44–49. doi: 10.1182/blood-2011-02-339275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. Cancer Facts and Figures, 2012. American Cancer Society. 2012 [Google Scholar]
- 12.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 14.Diamond C, Taylor TH, Im T, Wallace M, Saven A, Anton-Culver H. How valid is using cancer registries' data to identify acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma? Cancer Causes Control. 2007;18:135–142. doi: 10.1007/s10552-006-0096-5. [DOI] [PubMed] [Google Scholar]
- 15.Clarke CA, Glaser SL. Population-based surveillance of HIV-associated cancers: utility of cancer registry data. J Acquir Immune Defic Syndr. 2004;36:1083–1091. doi: 10.1097/00126334-200408150-00012. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. (23 ed) 2013 [Google Scholar]
- 17.World Health Organization. International Classification for Diseases. Geneva: Switzerland: 2010. [Google Scholar]
- 18.Surveillance Research Program. SEER*Stat software. National Cancer Institute. 2013 [Google Scholar]
- 19.Hessol NA, Katz MH, Liu JY, Buchbinder SP, Rubino CJ, Holmberg SD. Increased incidence of Hodgkin disease in homosexual men with HIV infection. Ann Intern Med. 1992;117:309–311. doi: 10.7326/0003-4819-117-4-309. [DOI] [PubMed] [Google Scholar]
- 20.Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119:3245–3255. doi: 10.1182/blood-2011-08-373738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Hodgkin Lymphoma. 2013 Report No.:2.2013. [Google Scholar]
- 22.Moyer VA. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013 doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros LJ, Greiner TC. Hodgkin's disease. Cancer. 1995;75:357–369. doi: 10.1002/1097-0142(19950101)75:1+<357::aid-cncr2820751318>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Rapezzi D, Ugolini D, Ferraris AM, Racchi O, Gaetani GF. Histological subtypes of Hodgkin's disease in the setting of HIV infection. Ann Hematol. 2001;80:340–344. doi: 10.1007/s002770100294. [DOI] [PubMed] [Google Scholar]
- 25.Mani H, Jaffe ES. Hodgkin lymphoma: an update on its biology with new insights into classification. Clin Lymphoma Myeloma. 2009;9:206–216. doi: 10.3816/CLM.2009.n.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hentrich M, Berger M, Wyen C, Siehl J, Rockstroh JK, Muller M, et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: results of a prospective multicenter study. J Clin Oncol. 2012;30:4117–4123. doi: 10.1200/JCO.2012.41.8137. [DOI] [PubMed] [Google Scholar]
- 28.Montoto S, Shaw K, Okosun J, Gandhi S, Fields P, Wilson A, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30:4111–4116. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]