Figure 2.
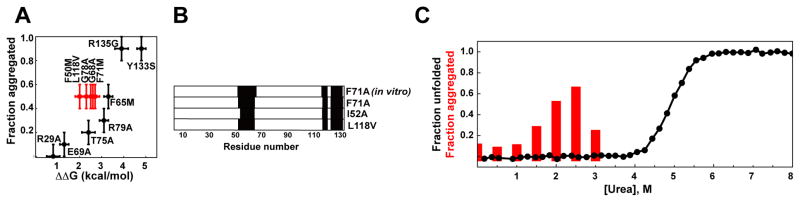
Origin of aggregation propensity in CRABP1 is more complex than simple increased fraction of the unfolded protein. A. Aggregation propensity (a fraction of insoluble protein) of different CRABP1 WT* mutants as a function of protein destabilization upon single point mutations (see also Table S1). B. Aggregation cores of different CRABP1 WT* aggregation-prone variants (F71A, I52A and L118V) obtained from E. coli inclusion bodies using DMSO-quenched H/D-exchange experiments and previously reported aggregation cores obtained in vitro using the F71A variant of CRABP1 WT*. (25) C. Aggregation propensity of CRABP1 WT* (red, monitored as a fraction of insoluble protein) and fraction of unfolded CRABP1 WT* (black, monitored by intrinsic tryptophan fluorescence spectroscopy) as a function of urea concentrations.
