Abstract
Virus vector -mediated gene transfer has been developed as a treatment for cystic fibrosis (CF) airway disease, a lethal inherited disorder caused by somatic mutations in the CFTR gene. The pathological pro-inflammatory environment of CF as well as the naïve and adaptive immunity induced by the virus vector itself limit the effectiveness of gene therapy for CF airway. Here, we report the use of an HDAC inhibitor, valproic acid (VPA), to enhance the activity of the regulatory T cells (Treg) and improve the expression of virus vector-mediated gene transfer to the respiratory epithelium. Our study demonstrates the potential utility of VPA, a drug used for over 50 years in humans as an anticonvulsant and mood-stabilizer, in controlling inflammation and improving the efficacy of gene transfer in CF airway.
Keywords: cystic fibrosis, gene therapy, VPA, Treg, airway, mouse
Introduction
Cystic fibrosis (CF) is a lethal inherited disorder caused by somatic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene in humans, affecting about 1 in 2,500 live births 1. Pathobiological symptoms of CF include muscosal obstruction of exocrine glands and pulmonary inflammation 2. Chronic neutrophilic inflammation and pulmonary infections are among the chief contributors to morbidity and mortality in patients with CF 3. Patients with CF have increased levels of proinflammatory cytokines in the airways that include TNF-α, IL-1, IL-6 and IL-8, and reduced levels of anti-inflammatory cytokines such as IL-10 4.
The success of gene therapy for CF airway disease is dependent on the efficient delivery and expression of the CFTR gene 5 in the cells of the respiratory epithelium 6–8. Virus (i.e. adenovirus, lentivirus and adeno-associated virus) vector-based gene transfer has been developed to correct the underlying Cl- secretion defect in the CF airway epithelium 9, 10. However, cellular and humoral immune responses to viral antigens and the epitopes on the expressed proteins have been shown to be barriers to efficient airway gene transfer 11, 12. Successful gene therapy regimens using virus-based vectors may also require the elimination of the anti-vector capsid immune responses 13–15. It is likely that two sets of antigens induce a host immune response, the viral antigens associated with the virus vector and the non-tolerated antigens of the newly expressed proteins.
Regulatory T cells (Treg) reduce pulmonary inflammation and lung injury in animal models of Pneumocystis pneumonia 16. Activated CD4+CD25+FOXP3+ Treg also suppress allergic airway inflammation 17. We are particularly interested in the post-translational modification of FOXP3 as mechanisms to regulate the activity of Treg 18, 19. Acetylation of FOXP3 has been shown to increase the stability of the FOXP3 proteins 19–21. Previously valproic acid (VPA), an HDAC inhibitor and a clinically safe compound, was shown to enhance the function of Treg to suppress effector cells 22. Here we report a strategy that utilizes VPA to improve virus vector-based gene transfer in the CF mouse lung. VPA treatment increased Treg activity and reduced inflammation in the CF mouse lung as demonstrated by the reduction of the number of neutrophils. Furthermore, following VPA treatment we observed an increase in adenovirus vector-mediated gene expression. This study suggests that HDAC inhibitors may be developed and used as immune-modulators to improve gene therapy.
Results
Increased number of Treg in the Bronchoalveolar lavage (BAL) fluid of CF mice
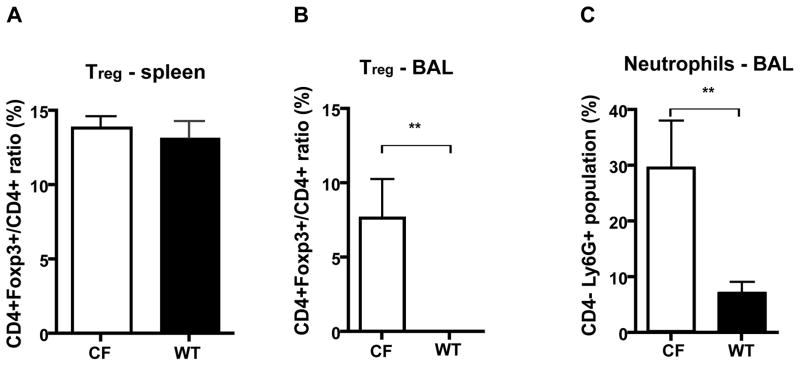
A CFTR knockout mouse model 23 has been used extensively to study CF-related disease and explore therapies. In our experiments to examine the role of Treg in the CF mouse lung, we compared the number of Treg in both CF and wild type (WT) age-matched mice. Splenocytes as well as cells isolated from the BAL fluid were harvested from CF and WT mice and analyzed for the presence of Treg by FACS. No difference was observed in the frequency of spleen-derived Treg between CF and WT mice. However, we did observe a higher percentage of Treg in the BALF of CF mice when compared to the WT mice (7.7% ± 2.6 v.s. 0% ± 0, Fig 1B), a likely consequence of the preexisting inflammatory status of the CF mouse. Compared to WT mice, CF mice had an increased number of Ly6G+ neutrophils (Fig 1C), which are myeloid cells shown to contribute to the innate immune defense against microbial pathogens 24.
Figure 1.
Comparison of Treg and neutrophil frequencies in CF and WT mice. (A) Splenocytes and (B, C) BAL fluid cells harvested from CF mice and WT mice were analyzed by FACS. The ratio of CD4+Foxp3+/ CD4+ reflects the frequency of Treg as the percentage of the CD4 positive population. Data are presented as the average of 3 mice. Error bar denotes SEM. **P < 0.01, T test. CF: cystic fibrosis, WT: wild type.
HDACi enhances adenovirus-mediated transgene expression in lung
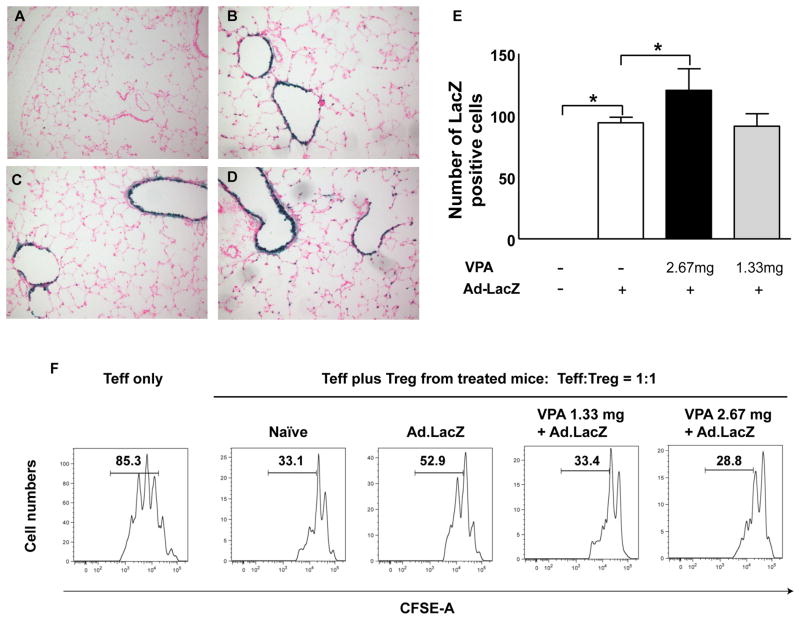
We first investigated if VPA could enhance adenovirus vector-mediated LacZ expression in mouse lung. CF mice were treated intraperitoneally with either low dose (1.33 mg) or high dose (2.67 mg) of VPA once daily for 4 days. On day 2 the mice were given intranasally (IN) 5x1010 particles of Ad.LacZ vector in 50μl. Expression of LacZ was inspected on day 5.
As expected, no transgene expression was observed in the lung of naïve mice. LacZ expressing cells were present in the lung of animals given the Ad.LacZ vector IN. Treatment with the high dose of VPA resulted in a significant increase of LacZ expression in mouse airway when compared to the Ad.LacZ vector only treated group (Figure 2E). No improvement was observed in the level of LacZ expression in the lung of mice treated with the low dose of VPA (Figure 2E).
Figure 2.
Adenovirus-mediated LacZ gene expression in CF mouse lung. CF mice were subjected to VPA treatment and dosed with 5x1010 of the Ad.LacZ vector IN. Representative images from (A) Naïve mice, (B) Ad.LacZ vector-treated mice, (C) Low dose VPA (1.33 mg daily) and Ad.LacZ vector-treated mice and (D) High dose VPA (2.67 mg daily) and Ad.LacZ vector-treated mice. (E) Quantification of LacZ positive cells in the lung of the Ad.LacZ vector treated mice. Values are presented as the average of 3 mice. Error bar denotes SD. *P < 0.05, Dunnett’s multiple comparison test. (F) Treg function in CF mice treated with the Ad.LacZ vector and VPA. CFSE-labeled Teff cells were isolated from the spleen and incubated with Treg at the indicated ratios. The proliferative fraction of Teff cells is shown. CF: cystic fibrosis, WT: wild type
VPA reduces inflammation in the lung of CF mice
In CF mice treated with either the low or high dose of VPA, there was a considerable reduction in the neutrophil population but not in Treg numbers (data not shown). To understand if the Treg activity was changed by the VPA treatment, CD4+CD25high Treg cells were isolated from the spleen and studied for their ability to suppress the proliferation of carboxyfluorescein diacetate succinimidyl ester (CFSE) –labeled primary CD4+CD25−CD45RBhigh T cells (representing effector T cells, Teff). Treatment with VPA dose-dependently increased Treg activity in the BAL fluid of CF mice that received the Ad.LacZ vector and less Teff underwent proliferation (Fig 2F).
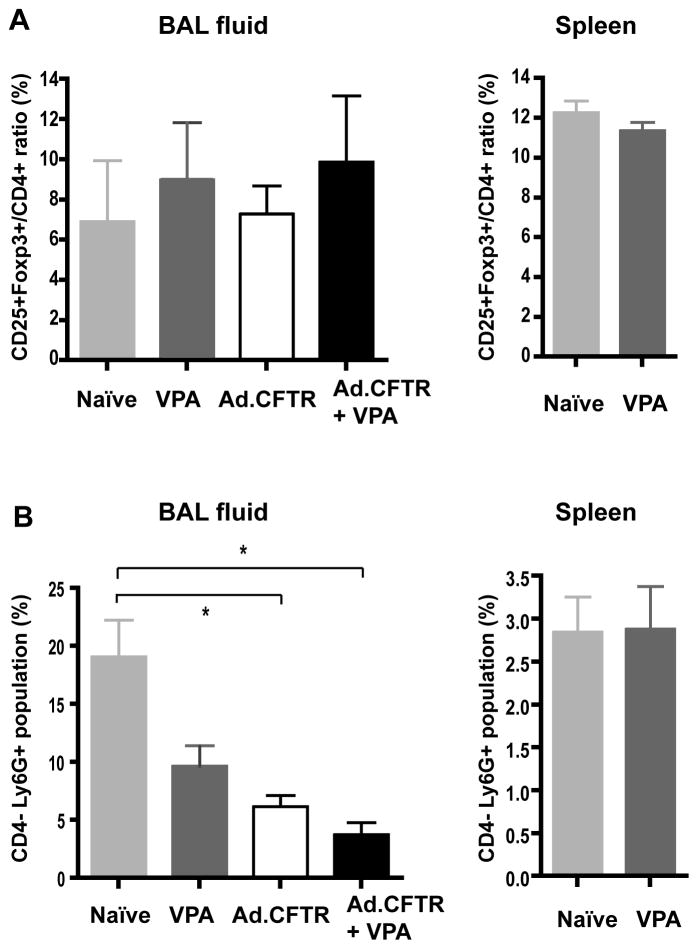
We then studied the effect of VPA on CFTR gene transfer. Independent of the Ad.CFTR vector, VPA treatment only slightly increased the percentage of Treg in the BAL fluid of CF mice (Figure 3A). The difference, however, was not statistically significant due to the significant variations between samples. Treatment with the Ad.CFTR vector alone did not appear to have an effect on the frequency of Treg in the BAL fluid. In contrast, VPA treatment or Ad.CFTR vector delivery led to a reduction in the frequency of neutrophils (Ly6G+) in the BAL fluid, suggesting less inflammation in these mice. VPA treatment combined with the Ad.CFTR vector delivery had an additive significant effect on the reduction of neutrophils compared to the naïve group (p <0.05) (Fig 3B). To rule out a possible toxic effect of VPA on neutrophils, we also examined the population of splenocytes from each experimental group. VPA treatment did not significantly affect the frequency of neutrophils and Treg frequency in spleen (Fig 3). Our data suggested that VPA treatment reduced BAL fluid neutrophil number by indirectly limiting inflammation and not by directly inducing cytotoxicity towards this population.
Figure 3.
In vivo effect of VPA. BAL fluid or spleen cells from mice subjected to different treatments as noted were collected for FACS analysis. Frequency of (A) Treg and (B) neutrophils was analyzed using FACS. * P < 0.05, t-test.
While the frequency of Treg was not changed (Fig 3A), we investigated whether the Treg activity was affected by either the VPA or the Ad.CFTR vector treatment. We examined Treg for their ability to suppress Teff cells as described above. Teff and Treg were mixed at a ratio of 2:1. The percentage of proliferative Teff cells was reduced from 75.4% (in the absence of Treg) to 57% by the addition of Treg from naïve CF mice (Fig. 4). In the presence of VPA-treated Treg, the population of proliferating Teff cells was further reduced to 48.1%, indicating an enhanced Treg activity in CF mice following VPA treatment (Fig. 4). Interestingly, Ad.CFTR vector treatment had little effect on Treg activity, and the effect of the combination of VPA and Ad.CFTR treatment was similar to the VPA only treatment (Fig. 4). These data are consistent with previous reported pro-Treg activity of VPA 22. We expect that that CFTR expression in lung epithelial cells by the Ad.CFTR vector would limit inflammation by restoring CFTR activity and not by increasing Treg activity. We speculate that by invoking two mechanisms the combination of the Ad.CFTR and VPA treatment may lead to lower inflammation as seen by the lower frequency of neutrophils in BAL fluid (Fig 3).
Figure 4.
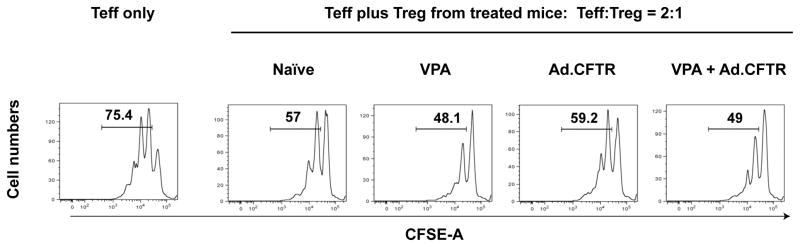
VPA enhances Treg function. CFSE-labeled Teff cells were co-cultured with Treg from CF mice that were treated with VPA, the Ad.CFTR vector or with the combination of VPA and the Ad.CFTR vector. Cells were analyzed by FACS, the proliferative fraction of Teff cells was calculated is presented.
Discussion
VPA has been shown to inhibit HDAC activity and affect the acetylation of histones and as such is expected to modulate gene transcription by promoting DNA decondensation 18, 25. However, previous studies have demonstrated that HDAC inhibitors alone are insufficient to broadly modify gene expression. In a microarray study, the pan-HDAC inhibitor Trichostatin (TsA) was shown to influence (equal distribution of up- or down-regulation) the transcription of ~2% genes in T cells26. In another study using the pan-HDAC inhibitor LAQ824, the Toll-like receptor 4- dependent activation of macrophages was examined and only 5% of genes were found to be either up- or down-regulated 27.
Fan and colleagues reported that treatment with VPA resulted in increased expression of exogenous genes in cells transduced with various viral-based gene transfer vectors, including adenovirus, adeno-associated virus and herpesvirus vectors 28. Recently, the effect of VPA on adenovirus vector-mediated transduction was reported. VPA concentrations as low as 1 mM increased adenoviral transduction of glioma cells by 7 fold 29. Although the pleiotropic effects of VPA may also contribute to the enhanced gene transfer in the airway observed in our study, the focus of our studies was the immunosuppressive effect of VPA through the regulation of Treg activity (Fig 2).
Inflammatory responses that arise following virus vector-mediated gene therapy in CF airway may involve cytotoxic T cells 13, T helper cells 30, and dendritic cells 31. CD4+CD25+FOXP3+ Treg suppress the function of all these immune cells 32. While Treg abnormalities have been reported in CF patients 33, the role of Treg in CF disease pathogenesis remains unclear.
We examined CD4+CD25+FOXP3+ Treg isolated from CF mice and observed an increase in the number of both Treg and neutrophils in BAL fluid. Our observations are consistent with previous reports of higher frequency of Treg at sites of ongoing chronic inflammation in lung 34 and the pathological influx of neutrophils in the airways of CF due to the loss of the CFTR function 35. In some CF patients, signs of inflammation, such as neutrophil accumulation, high concentration of IL-8 and abundance of free protease, is often observed in the airways even in the absence of an infection 36.
Despite the increased number of cells, the Tregs in BAL fluid were not sufficient to control the pulmonary inflammation in CF due to airway infection. We hypothesize that the HDAC inhibitor VPA functions as an immune suppressor by promoting FOXP3 acetylation as previously shown18 and enhancing the function of Treg, as demonstrated in Figures 2F and 4.
FOXP3 has an essential role in the development and function of natural and induced Treg and as such represents a key target to modulate Treg functions18, 37. Acetylation of FOXP3 is linked to stability of FOXP3 21, 38 that can be regulated by acetyltransferases (i.e. p300 and TIP60) and deacetylases (i.e. HDAC7, HDAC9 and SIRT1) 20, 37, 39. Recent studies suggest that in vivo treatment with VPA increases the number and function of CD4+CD25+FOXP3+ cells and reduces disease severity in the collagen-induced arthritis-animal model 22. In our study CF mice were characterized by highly elevated levels of Treg cells in the BAL fluid compared to WT mice (Fig 1B). Although VPA may further increase the frequency of Treg in CF mice, we propose that the ability of VPA to enhance Treg suppressive function is more critical and may instantly induce the pre-localized Treg.
Unexpectedly, we found that treatment with Adenovirus vector reduced Treg activity in CF mice. As shown in Fig. 2F, mice treated with only Ad.LacZ had much lower Treg activity than the control. It is unclear whether the induced inactivation of Treg function by Ad.LacZ vector is related to the influence of the virus vector on host immune system. Treatment with VPA prior to Ad.LacZ gene transfer restored Treg activity. We found that VPA treatment decreases neutrophil infiltration in the lung of CF mice (Figure 3B). Co-treatment of VPA with the Ad.CFTR vector further reduced the numbers of neutrophils in the BAL fluid of CF mice.
The capability of CD4+CD25+Foxp3+ Treg to reduce neutrophil survival and limit inflammatory response was reported in several models 43, 44. In a LPS induced lung injury model, alveolar infiltration of both Treg and neutrophils were observed after acute lung injury, and the transfer of Treg to injured mice enhanced the clearance of neutrophils from BAL fluid 44. Our data suggest that by inducing the activity of Treg, VPA appears to reduce neutrophils in the lung.
The clinical utility of Ad-based vectors for lung-gene therapy is severely limited by their immunogenicity and low transduction efficiency of airway epithelial cells 40. Compared with Ad-based vectors, adeno-associated virus (AAV)-based vectors are less inflammatory and thus favorable for repeat administration and long-term transgene expression. Furthermore, several AAV serotypes exist with improved targeting and transduction of airway epithelial cells 41, 42. Here, we demonstrate the use of the immune-suppressive HDAC inhibitor VPA to enhance transgene expression.
In summary, VPA may complement the effectiveness of CFTR gene transfer by inducing Treg activity in vivo. Our studies support further evaluation of VPA as a potential complimentary therapeutic to diminish inflammation in CF airway. Other HDAC inhibitors (e.g. vorinostat and romidepsin 45) that have been approved for clinical use can also be explored for their activity to facilitate virus vector-based gene transfer.
Materials and Methods
Animals studies
Studies utilizing mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Adenovirus-based vector at a dose of 5x1010 particles /mouse were delivered in 50μl of PBS IN as described previously 46. For the VPA plus the Ad.LacZ vector group, mice were treated daily with either 1.33 mg (low dose group) or 2.67mg (high dose group) delivered intraperitoneally (IP) for 4 consecutive days. The Ad.LacZ vector was dosed on the second day after VPA injection. On the fifth day, BAL fluid and lung tissue were collected. For the VPA plus the Ad.CFTR vector group, mice were first treated with 2 mg VPA or PBS by i.p. injection before and on the day of virus vector administration. Mice were further treated with 8 mg VPA three times over the period of one week after receiving the Ad.CFTR vector. Spleen cells and BAL fluid were collected for the analysis. PBS was used as the control treatment. Lungs from mice were inflated with 1:1 PBS/OCT, prepared as 8μm tissue sections and stained for LacZ expression. The slides were counterstained with Safranin O for LacZ expression 46.
Flow cytometry
Spleen cells and BAL fluid cells were collected and a single suspension of cells were incubated with 5% FCS containing PBS to block the Fc receptor. Cells were stained with anti CD4-FITC, CD25-PE (BD Pharmingen) and anti-Ly6G (Biolegend). After washing, cells were fixed and stained with anti Foxp3- APC (eBioscience) using Foxp3 staining buffer set (eBioscience). Flow cytometry was performed by LSRII (BD) at the University of Pennsylvania Flow Cytometry Core Facility.
In vitro Treg suppression assay
CD4+ T cells were isolated from the spleen of mice using the MACS CD4+ T cell isolation kit II (Miltenyi). CD4+CD25−CD45RBhigh Teff cells and CD4+CD25high Treg were isolated by FACS Aria II, yielding a purity of ~ 97% for both type of cells. Teff were labeled with CFSE (Invitrogen), stimulated with anti-CD3/CD28 beads (Invitrogen), and co-cultured with different ratio of Treg. After 3 days of co-culture in RPMI supplemented with 10% FBS, 1X non-essential amino acids (Invitrogen), 2mM sodium pyruvate (Invitrogen) and 50μM β-mercaptoethanol (Sigma), cells were harvested and in vitro proliferation of lymphocytes was analyzed by the FACSCanto Flow Cytometry.
Acknowledgments
We thank the members of the Vector Core, Animal Models Core and Cell Morphology Core of the Gene Therapy Program at the University of Pennsylvania for assistance with these studies. Flow cytometry was performed at the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource, a member of Path BioResource, in the Perelman School of Medicine of the University of Pennsylvania, which was established in part by equipment grants from the NIH Shared Instrument Program, and receives support from NIH P30 CA016520 from the National Cancer Institute. This work was supported by grants from the National Institutes of Health grants 5P30DK047757 (Pilot grant) and P01 AI073489-03.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- 1.Massie J, Curnow L, Gaffney L, Carlin J, Francis I. Declining prevalence of cystic fibrosis since the introduction of newborn screening. Archives of disease in childhood. 2010;95(7):531–3. doi: 10.1136/adc.2009.172916. [DOI] [PubMed] [Google Scholar]
- 2.Koehler DR, Downey GP, Sweezey NB, Tanswell AK, Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. American journal of respiratory cell and molecular biology. 2004;31(4):377–81. doi: 10.1165/rcmb.2004-0124TR. [DOI] [PubMed] [Google Scholar]
- 3.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. The New England journal of medicine. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, et al. Inflammatory cytokines in cystic fibrosis lungs. American journal of respiratory and critical care medicine. 1995;152(6 Pt 1):2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 5.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 6.Griesenbach U, Geddes DM, Alton EW. Gene therapy progress and prospects: cystic fibrosis. Gene therapy. 2006;13(14):1061–7. doi: 10.1038/sj.gt.3302809. [DOI] [PubMed] [Google Scholar]
- 7.Ziady AG, Davis PB. Current prospects for gene therapy of cystic fibrosis. Current opinion in pharmacology. 2006;6(5):515–21. doi: 10.1016/j.coph.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Prickett M, Jain M. Gene therapy in cystic fibrosis. Translational research : the journal of laboratory and clinical medicine. 2013;161(4):255–64. doi: 10.1016/j.trsl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347(6291):358–63. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- 10.Drumm ML, Pope HA, Cliff WH, Rommens JM, Marvin SA, Tsui LC, et al. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62(6):1227–33. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. Journal of virology. 1995;69(4):2004–15. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson JM. Adeno-associated virus and lentivirus pseudotypes for lung-directed gene therapy. Proceedings of the American Thoracic Society. 2004;1(4):309–14. doi: 10.1513/pats.200409-041MS. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Su Q, Wilson JM. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. Journal of virology. 1996;70(10):7209–12. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jooss K, Turka LA, Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene therapy. 1998;5(3):309–19. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 15.Sack BK, Herzog RW. Evading the immune response upon in vivo gene therapy with viral vectors. Current opinion in molecular therapeutics. 2009;11(5):493–503. [PMC free article] [PubMed] [Google Scholar]
- 16.McKinley L, Logar AJ, McAllister F, Zheng M, Steele C, Kolls JK. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. Journal of immunology. 2006;177(9):6215–26. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202(9):1199–212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Xiao Y, Zhu Z, Li B, Greene MI. Immune regulation by histone deacetylases: a focus on the alteration of FOXP3 activity. Immunology and cell biology. 2012;90(1):95–100. doi: 10.1038/icb.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du T, Nagai Y, Xiao Y, Greene MI, Zhang H. Lysosome-dependent p300/FOXP3 degradation and limits T cell functions and enhances targeted therapy against cancers. Exp Mol Pathol. 2013;95(1):38–45. doi: 10.1016/j.yexmp.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115(5):965–74. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 21.Song X, Li B, Xiao Y, Chen C, Wang Q, Liu Y, et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep. 2012;1(6):665–75. doi: 10.1016/j.celrep.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saouaf SJ, Li B, Zhang G, Shen Y, Furuuchi N, Hancock WW, et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol. 2009;87(2):99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257(5073):1083–8. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 24.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews Immunology. 2006;6(3):173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138(5):1019–31. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109(3):1123–30. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 28.Fan S, Maguire CA, Ramirez SH, Bradel-Tretheway B, Sapinoro R, Sui Z, et al. Valproic acid enhances gene expression from viral gene transfer vectors. Journal of virological methods. 2005;125(1):23–33. doi: 10.1016/j.jviromet.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Stedt H, Samaranayake H, Pikkarainen J, Maatta AM, Alasaarela L, Airenne K, et al. Improved therapeutic effect on malignant glioma with adenoviral suicide gene therapy combined with temozolomide. Gene therapy. 2013 doi: 10.1038/gt.2013.46. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari S, Griesenbach U, Geddes DM, Alton E. Immunological hurdles to lung gene therapy. Clinical and experimental immunology. 2003;132(1):1–8. doi: 10.1046/j.1365-2249.2003.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chirmule N, Gao G, Wilson J. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. Journal of virology. 2000;74(17):8003–10. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203(3):489–92. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahat N, Rivlin J, Iancu TC. Functional immunoregulatory T-cell abnormalities in cystic fibrosis patients. Journal of clinical immunology. 1989;9(4):287–95. doi: 10.1007/BF00918660. [DOI] [PubMed] [Google Scholar]
- 34.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29(1):114–26. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Conese M, Copreni E, Di Gioia S, De Rinaldis P, Fumarulo R. Neutrophil recruitment and airway epithelial cell involvement in chronic cystic fibrosis lung disease. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2003;2(3):129–35. doi: 10.1016/S1569-1993(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 36.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nature medicine. 2012;18(4):509–19. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Y, Li B, Zhou Z, Hancock WW, Zhang H, Greene MI. Histone acetyltransferase mediated regulation of FOXP3 acetylation and Treg function. Curr Opin Immunol. 2010;22(5):583–91. doi: 10.1016/j.coi.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Saouaf SJ, Samanta A, Shen Y, Hancock WW, Greene MI. Biochemistry and therapeutic implications of mechanisms involved in FOXP3 activity in immune suppression. Current opinion in immunology. 2007;19(5):583–8. doi: 10.1016/j.coi.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Greene MI. FOXP3 actively represses transcription by recruiting the HAT/HDAC complex. Cell Cycle. 2007;6(12):1432–6. [PubMed] [Google Scholar]
- 40.Conese M, Ascenzioni F, Boyd AC, Coutelle C, De Fino I, De Smedt S, et al. Gene and cell therapy for cystic fibrosis: from bench to bedside. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2011;10 (Suppl 2):S114–28. doi: 10.1016/S1569-1993(11)60017-9. [DOI] [PubMed] [Google Scholar]
- 41.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12993–8. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(2):294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards H, Williams A, Jones E, Hindley J, Godkin A, Simon AK, et al. Novel role of regulatory T cells in limiting early neutrophil responses in skin. Immunology. 2010;131(4):583–92. doi: 10.1111/j.1365-2567.2010.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119(10):2898–913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: can we interpret the code? Molecular oncology. 2012;6(6):637–56. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price A, Limberis M, Gruneich JA, Wilson JM, Diamond SL. Targeting viral-mediated transduction to the lung airway epithelium with the anti-inflammatory cationic lipid dexamethasone-spermine. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;12(3):502–9. doi: 10.1016/j.ymthe.2005.03.033. [DOI] [PubMed] [Google Scholar]