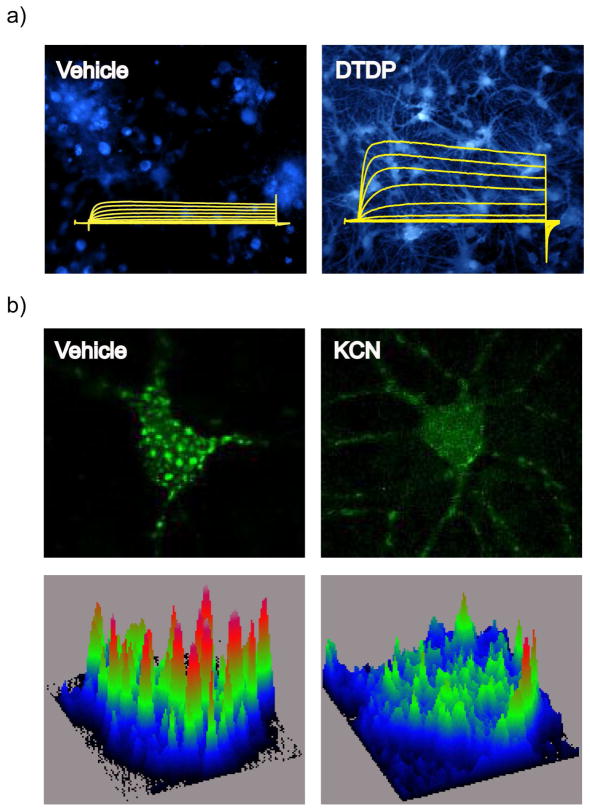
Fig. 1.
Kv2.1 channel-mediated pathways of neuronal apoptosis (right) and neuronal tolerance (left). (Right) An oxidant stimulus induces the release of Zn2+ from mitochondrial stores and metal-binding proteins, such as metallothionein (MT). Zn2+ activates ASK-1, leading to the phosphorylation and activation of p38 kinase. Zn2+ also inhibits PTPε and activates Src kinase. The combined action of both kinase systems results in increased phosphorylation of Kv2.1 channel residues S800 (by p38 kinase activation) and Y124 (by Src kinase activation and PTPε inhibition). Oxidant injury additionally stimulates release of Ca2+ from endoplasmic reticulum (ER) stores, which activates CaMKII. Coordinate phosphorylation of Kv2.1 channels at S800 and Y124, and the interaction of CaMKII with syntaxin, facilitate Kv2.1 channel-syntaxin binding, and subsequent channel delivery to the plasma membrane. Increased K+ currents through these newly inserted Kv2.1 channels permit the completion of the apoptotic signaling pathway by mediating cytoplasmic K+ loss. (Left) Neuronal activity or sub-lethal ischemia induces Ca2+ influx through glutamate receptors or intracellular Ca2+ release from the ER, and release of free Zn2+ from metal-binding proteins. Ca2+ increases calcineurin activity, leading to dephosphorylation and declustering of Kv2.1 channels. These changes are accompanied by a hyperpolarizing shift in the channel’s voltage-gated activation profile. Zn2+ is required for channel de-clustering and the voltage-gated activation shift, but not for Kv2.1 channel dephosphorylation. These changes in Kv2.1 channels reduce neuronal excitability in the context of an ischemic or epileptic insult, and render neurons tolerant to excitotoxic or other forms of injury