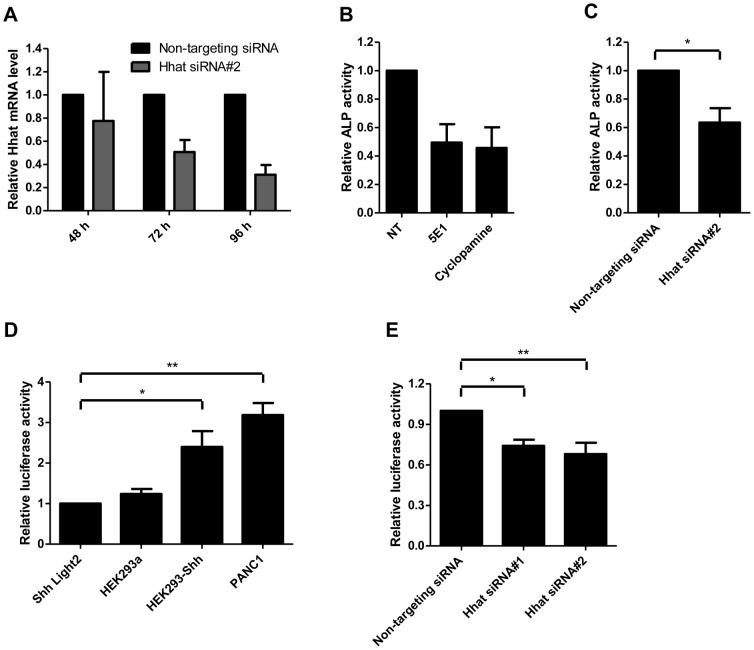
Figure 6. Hhat KD inhibits Hh-mediated juxtacrine/paracrine signaling.
A. Hhat-#2 siRNA KD in A549 cells was assessed by qPCR 24–96 h after siRNA transfection showing 20–60% decrease in Hhat mRNA expression. B. A549 cells co-cultured with C3H10T1/2 cells (1∶2 ratio) were treated with cyclopamine or 5E1 anti-Shh blocking antibody for 2 days and ALP induction in C3H10T1/2 cells was then determined using p-nitrophenyl phosphate in an ALP assay and measuring absorbance at 405 nm. C. A549 cells treated with Hhat-#2 siRNA for 2 days were co-cultured with C3H10T1/2 cells (1∶2 ratio) and ALP induction was assessed after additional 2 days as in B. The results show a significant reduction (*, P<0.05) in the ability of A549 cells treated with Hhat-#2 compared to cells treated with non-targeting (NT) siRNA pool to induce ALP production in C3H10T1/2 cells. The experiments were repeated four times and statistical significance was measured using the paired t test. D. PANC1 and HEK293a-Shh cells induce luciferase activity in the Shh-Light2 cells when co-cultured for 72 h, while the HEK29a cell line, which does not express Shh, did not show luciferase activity higher than background levels (Shh-Light2 cells alone). The data shown are representative of five independent experiments and are the means ± standard error of triplicates. Data were normalized to the Shh-Light2 cell monoculture response (*, P<0.05; **, P<0.01). E. PANC1 cells were transfected with Hhat-#1, Hhat-#2 or Non-targeting siRNAs. 48 h post-transfection, Shh-Light2 cells were mixed with the transfected cells (2∶1 ratio) and they were co-cultured for 72 h. The data shown are representative of three independent experiments and are the means ± standard error of triplicates. Data were normalized to the Non-targeting siRNA response (*, P<0.05; **, P<0.01).