Abstract
Acute graft-versus-host disease (aGVHD) occurs in 40-60% of recipients of partially matched umbilical cord blood transplantation (UCB). In a phase I study, adoptive transfer of expanded CD4+CD25+Foxp3+ natural regulatory T cells (nTregs) resulted in a reduced incidence of grade II-IV GVHD. To investigate potential mechanisms responsible for the reduced GVHD risk, we analyzed peripheral blood mononuclear cell (PBMC) mRNA expression of a tolerance gene set previously identified in operational tolerant kidney transplant recipients, comparing healthy controls to patients who received no nTregs or nTregs with and without GVHD. Samples from patients receiving nTregs regardless of GVHD showed increased Foxp3, but also B cell related tolerance marker expression. This correlated with early B cell recovery, predominately of naïve B cells, and nearly normal T cell reconstitution. CD8+ T cells showed reduced signs of activation (HLA-DR+ expression) in comparison to conventionally treated patients developing GVHD. In contrast, patients with GVHD had significantly increased whereas nTreg-treated patients without GVHD had reduced TLR5 mRNA expression. We identified Lin−HLADR−CD33+CD16+ cells and CD14++CD16− monocytes as main TLR5 producers especially in samples of conventionally treated patients developing GVHD. Together, these data reveal interesting similarities and differences between tolerant organ and nTreg-treated hematopoietic stem cell transplant recipients.
Keywords: hematopoetic stem cell transplantation, regulatory T cells, tolerance, graft versus host disease (GVHD), monocytes, toll-like receptor
Introduction
The use of UCB as an alternative source of hematopoetic stem cells (HSC) for patients with hematologic malignancies, who require a potentially curative allogeneic HSC transplant but lack a suitable related or unrelated adult donor, has grown tremendously (1). Although the risk for severe acute and chronic GVHD is lower relative to the degree of HLA mismatching, grade II acute GVHD in particular is still a common complication after UCB transplant, particularly in the setting of double UCB transplant (2-4). It is well described that the B cell recovery after UCB is faster as compared to e.g. unrelated bone marrow transplants (5). Conversely, delayed T cell reconstitution has been described after UCB (5). Early reconstitution of NK cells and CD4+ T cells following T cell-replete HSC has been associated with protection from transplant related mortality (6), whereas a slow T cell recovery is regarded as being primarily associated with deleterious infections, GVHD and disease relapse (7).
Thymus-derived CD4+25+ natural regulatory T cells (nTregs) are central for the maintenance of immune homeostasis and they can prevent allograft rejection (8). Clinical immunologists have thus strived to harness Tregs in novel tolerance-promoting strategies for the prevention of GVHD upon HSC transplantation, but also rejection after solid organ transplantation. Indeed, we previously demonstrated in a first-in-human clinical trial that infusion of polyclonally ex vivo expanded nTregs was associated with a apparent reduction in the incidence of grade II-IV GvHD with no demonstrable deleterious effect on the risks of infection, relapse, or early mortality in 23 nTreg-treated patients compared to 108 historical controls (1).
Recently, a set of genes was described, whose mRNA expression in PBMC distinguishes between tolerant kidney transplant recipients and patients with chronic rejection (9). The gene set contains three parameter groups. The first encompasses genes associated with Treg composition. Foxp3 as their master transcription factor is highly expressed by CD4+CD25+ Tregs (8), whereas expression of alpha-mannosidase (aMann) is increased in CD45RO+ memory T cells (10). Thus, the ratio of Foxp3 to aMann reflects the balance between Tregs and memory T cells. The second group encompasses genes, predominately or exclusively expressed by B cells such as CD20 (MS4A1), T-cell leukemia/lymphoma 1A (TCL1A, transcriptional regulator and AKT mediator abundantly expressed in naïve B cells, (11, 12), Fc receptor-like 1/Fc receptor like 2 (FCRL1/FCRL2, immunoregulatory transmembrane proteins, (13, 14)) and prepronociceptin (PNOC, opioid-like receptor (15)). The third group contains genes associated with composition or activation of innate immune cells such as toll-like receptor-5 (TLR5, pattern recognition receptor recognizing bacterial flagellin, (16)), heparan sulfate (glucosamine) 3-O-sulfotransferase 1 (HS3ST1, highly expressed by NK cells / dendritic cells (DCs) and mediating anti-inflammatory properties, (17)), SH2 domain containing 1B (SH2D1B=EAT-2, regulating NK cell cytotoxicity, (18, 19)) and solute carrier family 8 member 1 (SLC8A1=NCX1, regulating TNF-α production by monocytes (20)). The differences in gene expression between samples from tolerant and chronically rejecting kidney transplant patients reflected a relative and absolute increase of B cells, especially naïve (IgD+CD27−) and transitional (IgM+CD24+CD38++) B cells and controlled innate immune responses (9, 21).
We investigated whether the expression of the tolerance gene set might also reveal differences in recipients of nTreg with or without GVHD after UCB transplant. Interestingly, nTreg infusion, detectable for up to 2 weeks post-transplant, led to high Foxp3 mRNA expression in PBMCs analyzed 6 months post-transplant, regardless of the development of GVHD. This, in turn, was associated with nearly normal T cell frequencies as compared to healthy controls. Similarly the expression of B cell-related genes and reconstitution of especially naïve B cells was higher in PBMC samples from nTreg-treated patients. In contrast, expression of TLR5 was significantly different in nTreg-treated patients, regardless of GVHD occurrence. TLR5 mRNA and protein expression in PBMC was lowest in nTreg-treated patients without GVHD, whereas samples from conventionally treated patients regardless of GVHD showed the highest expression. Interestingly, we identified Lin−HLADR−CD33+CD16+ cells and CD14++CD16− monocytes to be the main TLR5 producers in samples from patients receiving conventional treatment. Therefore, Treg treatment appears to reduce the frequency and TLR5 expression of the former and partially the latter population. Thus, our data reveal overlapping features of tolerant solid organ transplant and nTreg-treated tolerant UCB transplant patients reflecting the restoration of a healthy “tolerant” balance between harmful and non-harmful leukocyte subpopulations.
Methods
Patient inclusion criteria
Patients with advanced or high-risk hematologic malignancy were eligible to receive UCB-derived Tregs if they met the following criteria: 18-70 years of age with an available HLA 4-6/6 UCB graft containing ≥ 3.0 × 107 nucleated cells/kg, suitable organ function for a nonmyeloablative regimen, and free of progressive fungal infection. In this study, all patients (with one exception) received two partially HLA matched UCB units as the HSC graft. Because of the potential increased risk of sustained dual chimerism after DUCBT, graft units were ABO-compatible as previously described (1). For this analysis, umbilical cord blood transplant patients were selected for analysis who had sufficient numbers of stored cells at 6 months post-transplant and were classified as having no GVHD versus grade I-IV acute GVHD and that did or did not receive supplemental Tregs as part of our phase I clinical trial, described below. No other criteria were used for patient selection.
UCB transplant and supportive care
All patients received a conditioning regimen consisting of cyclophosphamide 50 mg/kg on day −6, fludarabine 40 mg/m2 daily on days −6 to −2, and a single fraction of TBI 200 cGy without shielding on day −1. All patients received UCB followed by granulocyte-colony stimulating factor (Neupogen; Amgen) 5 μg/kg daily starting on day +1 until an absolute neutrophil count > 2500/μL was observed for 2 consecutive days. The second UCB unit was infused within 30 minutes of the first UCB unit infusion. The study was approved by the Institutional Review Board, registered by ClinicalTrials.Gov and supportive care was applied following guidelines as previously reported (1).
GVHD prophylaxis with Treg infusion and pharmacological agents
Patients were treated with 30 × 105 nTregs/kg actual body weight on day +2 (see Table 1; (1)). No patient developed a dose-limiting toxicity (DLT). Patients received mycophenolate mofetil (MMF) at 1.5 g intravenously or orally twice daily from day −3 to +30 in combination with cyclosporine (CsA) twice daily with target trough levels of 200-400 ng/mL. Because CsA has been shown to potentially interfere with optimal Treg function and survival (22-24), the last cohort received Tregs in the presence of sirolimus rather than CsA in combination with mycophenolate mofetil (MMF). Sirolimus was given with a loading dose of 12 mg followed by 4 mg daily and a target trough level between 3-12 μg/mL from day −3 to day +100. Tapering was accomplished during the course of 8-12 weeks unless GVHD was diagnosed.
Table 1.
Patient characteristics
| Patient | nTreg dose (day of infusion) |
GVHD prophylaxis |
max. GVHD grade (day of onset) |
GVHD organs | Chronic GVHD (onset) |
|---|---|---|---|---|---|
| noTregs / noGVHD #1 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #2 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #3 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #4 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #5 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #6 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #7 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #8 | None | Rapa/MMF | No | No | No |
| noTregs / noGVHD #9 | None | CsA/MMF | No | No | No |
| noTregs / noGVHD #10 | None | CsA/MMF | No | No | No |
| noTregs / GVHD #1 | None | CsA/MMF | 3 (14) | skin, lower gi | Yes (125) |
| noTregs / GVHD #2 | None | CsA/MMF | 3 (44) | lower gi | No |
| noTregs / GVHD #3 | None | CsA/MMF | 3 (27) | lower gi, upper gi | No |
| noTregs / GVHD #4 | None | CsA/MMF | 2 (37) | skin | No |
| noTregs / GVHD #5 | None | CsA/MMF | 2 (28) | skin | Yes (104) |
| noTregs / GVHD #6 | None | CsA/MMF | 3 (29) | skin, lower gi | No |
| noTregs / GVHD #7 | None | CsA/MMF | 3 (21) | skin, lower gi, upper gi | Yes (133) |
| noTregs / GVHD #8 | None | CsA/MMF | 3 (49) | skin, lower gi | No |
| noTregs / GVHD #9 | None | CsA/MMF | 4 (44) | skin, lower gi | No |
| Tregs / noGVHD #1 | 3×106 (2) | CsA/MMF | No | No | No |
| Tregs / noGVHD #2 | 3×106 (2) | Rapa/MMF | No | No | No |
| Tregs / noGVHD #3 | 3×106 (2) | Rapa/MMF | No | No | No |
| Tregs / noGVHD #4 | 3×106 (2) | Rapa/MMF | No | No | No |
| Tregs / noGVHD #5 | 3×106 (2) | Rapa/MMF | No | No | No |
| Tregs / noGVHD #6 | 3×106 (2) | CsA/MMF | No | No | No |
| Tregs / GVHD #1 | 3×106 (1) | CsA/MMF | 2 (41) | skin | No |
| Tregs / GVHD #2 | 3×106 (2) | CsA/MMF | 2 (37) | skin | Yes (1009) |
| Tregs / GVHD #3 | 3×106 (2) | CsA/MMF | 2 (29) | skin | No |
| Tregs / GVHD #4 | 3×106 (2) | CsA/MMF | 2 (21) | skin | No |
| Tregs / GVHD #5 | 3×106 (2) | Rapa/MMF | 3 (86) | skin, lower gi | No |
| Tregs / GVHD #6 | 3×106 (2) | CsA/MMF | 2 (39) | skin, upper gi | No |
gi=gastrointestinal tract
Manufacture of nTregs
nTregs were isolated from a partially HLA-matched third UCB unit (provided by The New York Blood Center) that was 4-6/6 HLA matched to the patient. Donor suitability was determined by current good manufacturing practices. Institutional standard operating procedures were followed to avoid cross-contamination. The UCB unit was thawed and processed according to standard procedures (1, 25). Enrichment, culture and expansion of nTregs was accomplished as previously published (1).
Quantitative RT-PCR (qRT-PCR) of tolerance set genes
Total RNA from PBMCs of patients collected 6months and one year after UCB was isolated using the Nucleospin RNAII kit (Machery-Nagel, Düren, Germany). 200ng of whole blood total RNA was reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) and synthesized cDNA was subjected to RT-PCR analysis. Quantitative RT-PCR was performed for the following genes using pre-made TaqMan® Gene Expression Assays from Applied Biosystems: Hs01099196_m1 heparan sulfate (glucosamine) 3-O-sulfotransferase 1 (HS3ST1), Hs01592483_m1 SH2 domain containing 1B (SH2D1B), Hs00172040_m1 Tcell leukaemia/lymphoma 1A (TCL1A), Fc receptor-like 2 (FCRL2), Fc receptor-like 1 (FCRL1), membrane-spanning 4-domains subfamily A member 1 = CD20 (MS4A1), (CD79B), prepronociceptin (PNOC), toll-like receptor 5 (TLR5) and solute carrier family 8 member 1 (SLC8A1) or self-designed panels: forkhead box P3 (Foxp3), Mannosidase I (aMann) and hypoxanthine guanine phosphoribosyl transferase (HPRT). HPRT was used as a house-keeping gene. Gene expression was determined applying the ΔΔCT method as previously described (9, 26).
Flow Cytometry
Thawed PBMC, collected at the same time as qRT-PCR samples, were washed and resuspended at 1 × 106/mL. Titrated amounts of fluorochrome-conjugated monoclonal antibodies were used to identify the following leucocyte subsets, CD45+CD14− for lymphocytes, CD3+ for T cells, CD19+ for B cells, CD56+/−CD16+CD3- for NK cells, CD4+CD3+HLA-DR+/− for CD4 T cells, CD8+CD3+HLA-DR+/− for CD8 T cells, CD14++/dimCD16−/+ for monocytes and CD3−CD19−CD56−CD14−HLADR+ for DCs. Antibody conjugates were obtained from BD Biosciences (Heidelberg, Germany) or Beckman Coulter (Krefeld, Germany). Samples were co-stained with anti-TLR5-FITC (IMG-663C, Imgenex, San Diego, USA) using the cytofix/cytoperm staining solutions (BD Biosciences, Heidelberg, Germany). B cell subsets were defined as follows: IgD+CD27− naive B cells, IgD−IgM−CD27+ memory B cells and IgM+CD24+CD38++ transitional B cells. Cells were fixed with 1% paraformaldehyde/PBS and data acquired on a BD LSRII within 24 hours.
Statistical analysis
Non-parametric tests were used to estimate statistical significance as the patient numbers were low and data did not conform to a normal distribution. Wilcoxon signed rank test was used to compare responses within the same group of patients. Mann-Whitney U tests were used to compare medians between patients groups.
Results
Clinical outcomes
Treg dose, GVHD prophylaxis and grading characteristics of analyzed patient groups are shown in table 1. All patients, who did not receive nTregs, were treated with CsA for GVHD prophylaxis. Of the patients studied, the majority of nTreg-treated patients without GVHD (4 out of 6) received Sirolimus for GVHD prophylaxis, whereas nearly all (5 out of six) nTreg-treated patients with GVHD were treated with CsA.
High PBMC Foxp3 and B cell-related gene expression levels in patients without GVHD and their relationship to nTreg therapy
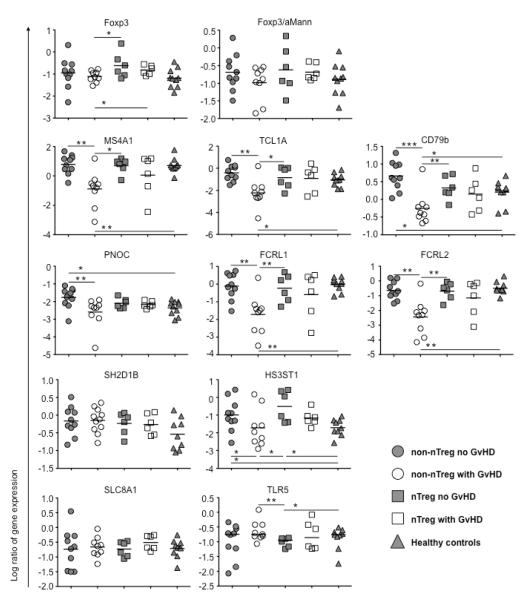
PBMC samples were collected at 6 months post-transplant from the following patient groups: non-nTreg patients without GVHD (n=10), non-nTreg patients with GVHD (n=9), nTreg-treated patients without GVHD (n=6), nTreg-treated patients with GVHD (n=6), and PBMC samples from healthy controls (n=10). These samples were analyzed for mRNA expression of the recently described indices of tolerance (IOT) gene set comparing group 1 (Treg-associated genes), group 2 (B-cell-associated genes) and group 3 (genes associated with innate immune cell function) biomarkers (9).
With respect to the group 1 biomarker FoxP3, there were significantly higher FoxP3 mRNA levels in PBMC of nTreg treated patients without GVHD as compared to non-nTreg treated patients with GVHD at 6 months post-UCB transplant (Figure 1). As we have previously reported we can only find infused nTregs for up to 14 days post-infusion (1). Thus, we interpret the high FoxP3 expression to be an indirect effect of GVHD elimination in the context of nTreg infusion.
Figure 1. Expression of transplant-tolerance-associated genes.
MRNA expression of IOT genes was analyzed in PBMCs of patients receiving UCB under conventional immunosuppressive treatment developing no GVHD (non-nTreg no GVHD, n=10), patients receiving UCB under conventional immunosuppressive treatment developing GVHD (non-nTreg with GVHD, n=9) or additional nTreg transfer with (nTreg with GVHD, n=6) or without development of GVHD (nTreg no GVHD, n=6) at 6 months post-transplant, and of healthy controls (n=10) by qRT-PCR as described in material and methods. Data are shown as log transformed gene expression values calculated in relation to the house keeping gene HPRT, *p≤0.05, **p≤0.01, ***p≤0.001
Samples from nTreg-treated patients without aGVHD also displayed increased expression of B cell related genes such as MS4A1, TCL1A, CD79b, PNOC, FCRL1 and FCRL2 as compared to samples from non-nTreg, conventionally treated patients developing GVHD (Figure 1). Because conventionally patients without GVHD had similar increased early expression of B cell related genes as nTreg-treated patients without GVHD, the observed solid organ B cell tolerance signature was reflective of GVHD status and not of nTreg infusion per se.
Next, we investigated whether the high expression of Foxp3 and B cell related genes in samples from nTreg-treated patients without GVHD were due to differences in immunosuppressive maintenance therapy. Expression results from samples of nTreg-treated patients, regardless as to whether or not acute GVHD had been observed, were plotted according to CsA- or sirolimus-based maintenance therapy (Supplementary Figure 1). The results clearly show that the expression of Foxp3 was similar between samples from nTreg-treated patients on CsA- or Sirolimus-based maintenance therapy. Expression of MS4A1, FCRL1 and FCRL2 was reduced in samples of some CsA-treated patients. However this did not reach statistical significance and was only seen for three patients, who all developed GVHD.
Decreased TLR5 mRNA expression in PBMC of nTreg-treated patients without GVHD
With regard to group 3 biomarkers no differences between groups were seen in expression of SH2 domain containing 1B (SH2D1B=EAT-2, regulating NK cell cytotoxicity, (18, 19)) and solute carrier family 8 member 1 (SLC8A1=NCX1, regulating TNF-α production by monocytes (20)) (Figure 1). In contrast, we observed increased mRNA expression at 6 months post-UCB transplant in PBMC for HS3ST1 (3-O-sulfotransferase 1, highly expressed by NK cells / CD33+ DCs and mediating anti-inflammatory properties) expression in samples from either nTreg- or non-nTreg- treated patients not developing GVHD as compared to healthy controls or conventionally treated GVHD patients (Figure 1). Although nTreg infusion did not further increase HS3ST1 levels in no GVHD patients, nTreg treated patients with GVHD had HS3ST1 levels that trended higher (p=0.06) than conventionally treated patients developing GVHD, suggesting an effect of prior nTreg infusion on HS3ST1 levels.
Interestingly, we detected decreased TLR5 expression in samples from nTreg-treated patient who did not develop GVHD as compared to non-nTreg patients with GVHD (Figure 1). Only in nTreg-treated patients without GVHD did the TLR5 expression normalize, reaching levels significantly lower compared to healthy controls. Again we investigated whether this was also influenced by the sirolimus-based maintenance therapy. Indeed, expression of TLR5 was increased in samples from some CsA-treated patients (Supplementary Figure 1). However this again did not reach statistical significance and was only seen for three patients, who all developed GVHD and in none of the four patients who did not develop GVHD. These data suggest that in GVHD-free patients nTreg infusion coupled with sirolimus- or CsA-based maintenance therapy had sufficient tolerization to normalize TLR5 expression levels.
Conventionally treated patients with GVHD have a low frequency of adaptive with a high frequency of innate immune cells
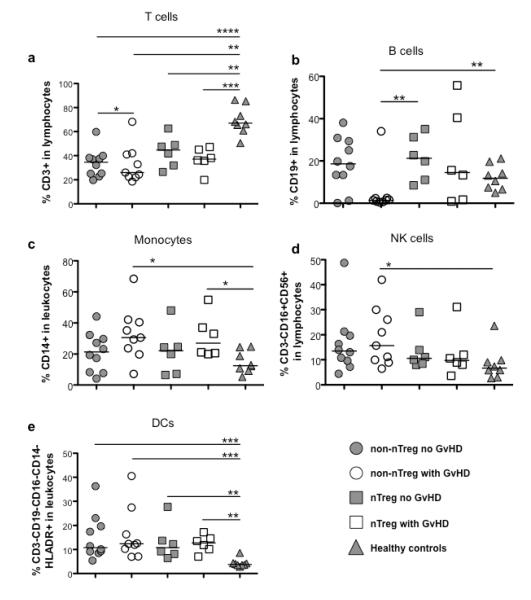
As the tolerance gene set identified in tolerant solid organ transplant patients went along with an altered composition of blood leukocyte subsets, we also studied whether nTreg treatment results in a modified leukocyte reconstitution upon UCB. The leukocyte subset distribution in samples of UCB transplant patients and healthy controls is shown in Figure 2. Samples from all transplant patients regardless of group showed reduced T cell frequencies compared to non-transplant controls (Figure 2a). However, samples from conventionally treated patients developing GVHD had the lowest frequency of T cells. Treatment with nTregs resulted in a higher absolute number of CD3+ T cells, especially in comparison to conventionally treated patients developing GVHD (Supplemental Figure 2). Total NK cell frequencies were highest in samples from conventionally treated patients developing GVHD (Figure 2d), though absolute numbers were comparable between all groups. Samples from both patient groups with GVHD contained more monocytes early after transplantation as compared to healthy controls but not significantly higher compared to samples from patients not developing GVHD (Figure 2c). The relative proportion of DCs was increased in all patient groups compared to healthy controls (Figure 2e). However, we detected no differences between the patients groups either with regard to the total DC population nor myeloid or plasmacytoid subpopulations (data not shown). Lastly, we detected normal B cell frequencies and absolute numbers in all patient groups except conventionally treated patients developing GVHD (Figure 2b).
Figure 2. Distribution of leukocyte subsets in peripheral blood.
Frequencies of T cells (a, CD3+), B cells (b, CD19+), monocytes (c, CD14+), NK cells (d, CD3−, CD19−, CD16+) and dendritic cells (e, CD3-CD19-CD16-CD14-HLADR+) in PBMCs of patients receiving UCB under conventional immunosuppressive treatment developing no GVHD (non-nTreg no GVHD, n=10), patients receiving UCB under conventional immunosuppressive treatment developing GVHD (non-nTreg with GVHD, n=9) or additional nTreg transfer with (nTreg with GVHD, n=6) or without development of GVHD (nTreg no GVHD, n=6) at 6 months post-transplant, and of healthy controls (n=10) were determined as described in material and methods. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001
Reversed balance of naïve and memory B cells in conventionally treated patients with GVHD
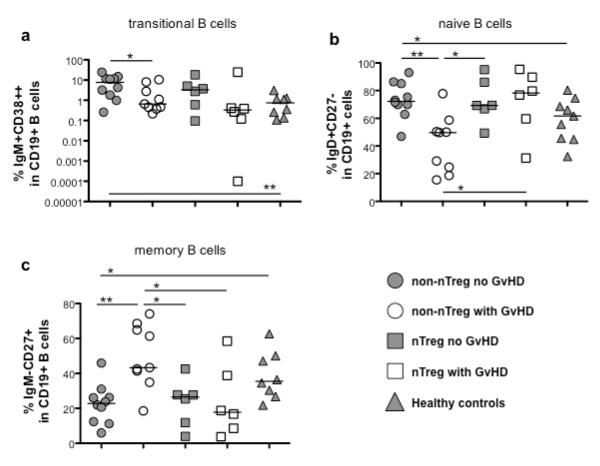
As the expression of B cell related genes from the IOT tolerance gene set was lowest in conventionally treated UCB transplant patients developing GVHD and also their absolute total B cell count was dramatically reduced, we investigated, whether the distribution of B cell subsets was also different. We analyzed the frequencies of transitional (Figure 3a, CD19+IgM+CD38++) as well as naive (Figure 3b, CD19+IgD+CD27−) and memory (Figure 3c, CD19+IgM−CD27+) B cells within the CD19+ cell compartment. B cells from conventionally treated transplant patients developing GVHD showed frequencies of naïve B cells that were significantly reduced, whereas the frequencies of memory B cells were increased compared to all other patient groups. In contrast, nTreg-treated patients that developed GVHD showed similar frequencies of naïve and memory B cells, compared to patients without GVHD. The relative proportions of transitional B cells were increased in samples from patients free of GVHD regardless of nTreg treatment.
Figure 3. Distribution of B cell subsets in peripheral blood.
Frequencies of transitional B cells (a, CD19+IgM+CD38++), naïve B cells (b, CD19+IgD+CD27−) and memory B cells (c, CD19+IgM−CD27+) in PBMCs of patients receiving UCB under conventional immunosuppressive treatment developing no GVHD (non-nTreg no GVHD, n=10), patients receiving UCB under conventional immunosuppressive treatment developing GVHD (non-nTreg with GVHD, n=9) or additional Treg transfer with (nTreg with GVHD, n=6) or without development of GVHD (nTreg no GVHD, n=6) at 6 months post-transplant, and of healthy controls (n=10) were determined as described in material and methods. *p≤0.05, **p≤0.01
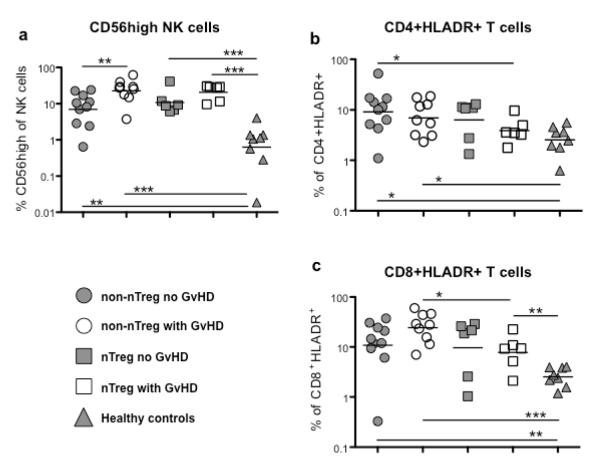
Impact of nTreg treatment on CD56high NK cells and activated HLA-DR+ T cells
We also studied whether transfer of nTregs affected the number of CD56high NK cells, that produce abundant cytokines, and activated T cells. As shown in figure 4a samples from all patient groups contained more CD56high NK cells compared to samples from healthy controls. This was most dramatic for conventionally treated patients developing GVHD but was not be sufficiently influenced by nTreg transfer. To study differences in numbers of activated T cells we assessed the frequencies of HLA-DR+ CD4+ and CD8+ T cells as such was done previously following stem cell transplantation (27-29). UCB transplant patients are characterized by higher percentages of activated, HLA-DR+ cells within the CD4+ and CD8+ T cell compartments compared to samples from healthy controls though statistical significance was reached only for non-Treg patients (Figure 4b and c). For CD4+ T cells this was largely independent of the development of GVHD. In contrast, samples from patients receiving conventional treatment who developed GVHD contained the highest proportion of HLA-DR+ CD8+ T cells. Interestingly, nTreg transfer seemed to counteract GVHD-associated generation of HLA-DR+ CD8+ T cells despite development of GVHD.
Figure 4. Distribution of NK and T cell subsets in peripheral blood.
Frequencies of CD56bright NK cells (a, CD3−CD16dimCD56high) and activated CD4+ (b, CD4+HLADR+) and CD8+ T cells (c, CD8+HLADR+) in PBMCs of patients receiving UCB under conventional immunosuppressive treatment developing no GVHD (non-nTreg no GVHD, n=10), patients receiving UCB under conventional immunosuppressive treatment developing GVHD (non-nTreg with GVHD, n=9) or additional nTreg transfer with (nTreg with GVHD, n=6) or without development of GVHD (nTreg no GVHD, n=6) at 6 months post-transplant, and of healthy controls (n=10) were determined as described in material and methods. *p≤0.05, **p≤0.01, ***p≤0.001
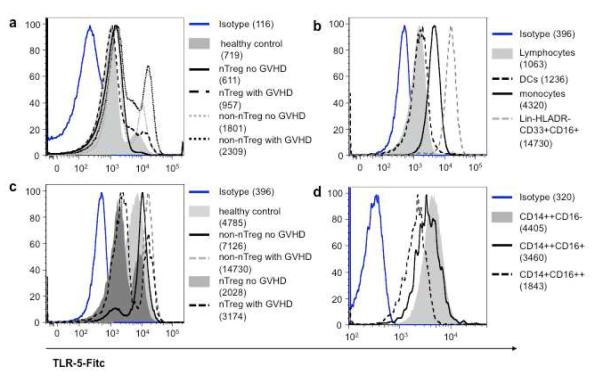
Identification of Lin−CD33+ granulocyte-like and CD16− monocytes as main TLR5 producers
Next, we sought to determine which leukocyte subset contributes to the increased TLR5 expression in patients developing GVHD. TLR5 staining of PBMCs from a conventionally treated patient developing GVHD revealed bimodal expression (Figure 5a) with monocytes and especially a subset of CD3−CD19−CD56−CD14−HLA-DR−CD33+CD16+ cells expressing higher levels as compared to lymphocytes and DCs (Figure 5b). As indicated by their surface expression the latter population is distinct from lymphocytes and monocytes (CD3-CD19−CD56−CD14−=lineage negative) but also from DCs (HLA-DR−). Interestingly, the TLR5 expression of CD3−CD19−CD56−CD14−HLA-DR−CD33+CD16+ cells was highest in samples from conventionally treated patients regardless of GVHD, whereas a large proportion of this cell subset showed low TLR5 expression in samples from nTreg-treated patients (Figure 5c; see also Figure 7). Furthermore, the percentage and phenotype of those CD3-CD19−CD56−CD14−HLA-DR−CD33+CD16+ cells are different in samples from nTreg-treated vs conventionally treated patients (Supplementary Figure 3). The numbers are reduced and they are characterized by higher CD16 expression as compared to samples from conventionally treated patients. Indeed, subgating revealed that the CD16low expressing cells are the main TLR5 expressing cells (data not shown). Peripheral monocytes have been subdivided into three subpopulations based on the relative expression level of CD14 and the presence of CD16, namely CD14++CD16−, CD14++CD16+ and CD14+CD16++ monocytes (30). These monocyte subsets have different functional properties, with CD14++CD16− and CD14++CD16+ being more inflammatory and the CD14+CD16++ monocytes displaying patrolling properties (31). Our subset analyses revealed that particularly the inflammatory CD14++CD16− monocytes expressed high amounts of TLR5 (Figure 5d; see also Figure 7).
Figure 5. TLR5 expression in leukocyte subsets.
Shown are representative histogram plots of TLR5 expression in whole PBMCs (a), lymphocytes versus CD14+ monocytes, dendritic cells and CD3-CD19-CD56-CD14-HLA-DR CD33+CD16+ cells (b), CD3-CD19-CD56-CD14-HLA-DR-CD33+CD16+ cells from representative samples of all patient groups and healthy controls (c) and CD14++CD16− versus CD14++CD16+ and CD14+CD16++ monocytes (d) measured as described in material and methods.
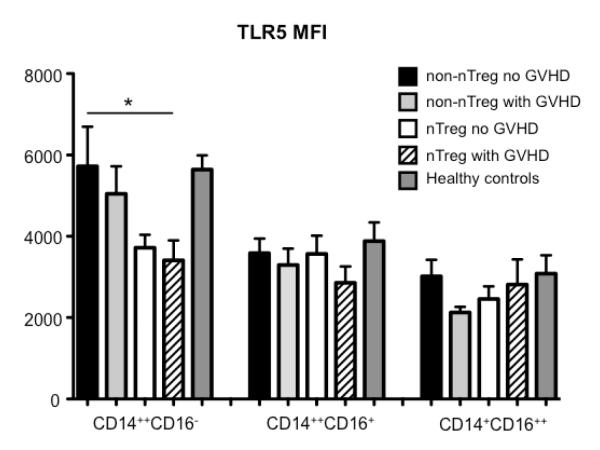
Figure 7. TLR5 expression in individual leukocyte subpopulations.
TLR5 expression depicted as mean fluorescence intensity in monocyte subsets from PBMCs of patients receiving UCB under conventional immunosuppressive treatment developing no GVHD (non-nTreg no GVHD, n=10), patients receiving UCB under conventional immunosuppressive treatment developing GVHD (non-nTreg with GVHD, n=9) or additional nTreg transfer with (nTreg with GVHD, n=6) or without development of GVHD (nTreg no GVHD, n=6) at 6 months post-transplant, and of healthy controls (n=10) were determined as described in material and methods. *p≤0.05
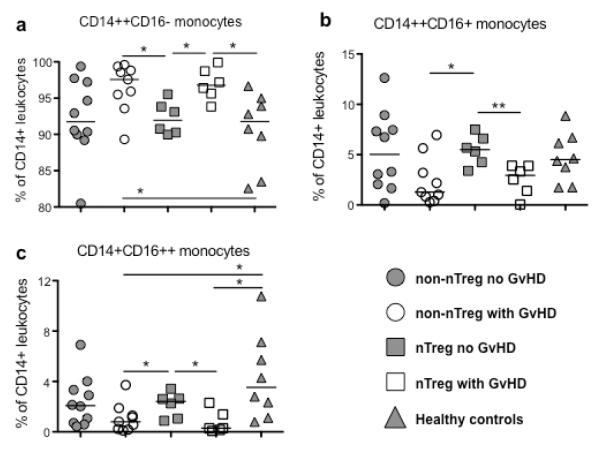
Reduced CD16− monocytes in samples from nTreg-treated patients without GVHD
In PBMC of transplant patients developing GVHD we detected significantly increased frequencies of CD14++CD16− monocytes compared to patients developing no GVHD and healthy controls (Figure 6a). In contrast, frequencies of both CD16 expressing monocyte subsets were significantly decreased in samples of those patients (Figure 6b and c).
Figure 6. Distribution of monocyte subpopulations.
Frequencies of CD14++CD16− (a), CD14++CD16+ (b) and CD14+CD16++ monocytes (c) in PBMCs of patients receiving UCB under conventional immunosuppressive treatment developing no GVHD (non-nTreg no GVHD, n=10), patients receiving UCB under conventional immunosuppressive treatment developing GVHD (non-nTreg with GVHD, n=9) or additional nTreg transfer with (nTreg with GVHD, n=6) or without development of GVHD (Treg no GVHD, n=6) at 6 months post-transplant, and of healthy controls (n=10) were determined as described in material and methods. *p≤0.05, **p≤0.01
Next we studied the TLR5 surface expression of all three monocyte subsets in samples from UCB transplant patients and healthy controls (Figure 7). As already described in figure 5 CD14++CD16− monocytes in general showed the strongest TLR5 expression. Interestingly, CD14++CD16− monocyte subsets from both nTreg-treated patient groups displayed the lowest TLR5 expression.
Again we investigated whether composition of monocyte subsets and TLR5 protein expression was also influenced by the sirolimus-based maintenance therapy. Although samples from nTreg-treated patients receiving CsA-based maintenance therapy contained by tendency more CD14++CD16− monocytes, this did not reach statistical significance (Supplementary Figure 4a). Furthermore, CD16++ monocytes from nTreg-treated patients receiving sirolimus-based maintenance therapy contained lower but not significantly TLR5 expression (Supplementary Figure 4b).
Discussion
Analyzing a recently described IOT tolerance gene set, our data showed, that nTreg treatment resulted in high Foxp3 expression in PBMC associated with a better reconstitution of T cells following UCB transfer especially in patients who did not develop GVHD (Figures 1, 2 and Supplementary Figure 2). Only samples from conventionally treated patients developing GVHD displayed reduced expression levels of B cell-related IOT genes such as MS4A1 and TCL1A (Figure 1). This was not observed in samples from nTreg-treated patients developing GVHD. In conventionally treated patients with GVHD, there was reduced relative and absolute B cell numbers and a shift from the naïve to memory phenotype prevented by nTreg infusion (Figures 2, 3 and Supplementary figure 2). Samples from conventionally treated patients developing GVHD contained more TLR5, which was expressed at the highest levels on CD3−CD19−CD56−CD14−HLA-DR−CD33+CD16+ cells and CD14++CD16− monocytes (Figures 1, 5, 6, 7 and Supplementary Figures 2 and 3), whereas samples from patients receiving nTregs who did not develop GVHD showed the lowest TLR5 expression, even lower as compared to healthy controls.
It was reported previously, and we confirm this in our report, that stem cell transplant patients developing GVHD had significantly lower numbers of naïve B cells, whereas levels of CD27 expressing memory / activated B cells were increased (32-35). We also detected an increased proportion of CD56high NK cells in conventionally treated patients developing GVHD. Samples of those patients contained also lower number of T cells. Indeed, Vukicevic and colleagues detected increased numbers of such NK cells following hematopoetic stem cell transplantation in patients with low T cell numbers (36).
TLR5 expression, reported to be increased in samples from chronically rejecting kidney transplant patients, was significantly reduced in nTreg-treated UCB patients without GVHD (Figures 1 and 3). Thus, low TLR5 expression was seen in both tolerant solid organ as well as nTreg-treated UCB transplant patients without GVHD. We performed an extensive TLR5 expression analysis in leukocyte subsets, which has never been done before. We identified CD3−CD19−CD56−CD14−HLA-DR−CD33+CD16+ cells and monocytes, in particular CD14++CD16− monocytes, to be the highest TLR5 expressing leukocyte subset (Figure 5). Of the former population especially the CD16low subset (Figure 5 and data not shown) was characterized by high TLR5 expression. Indeed, samples from conventionally treated patients contain more of these CD16low cells (Supplementary Figure 3). The CD33+CD16low cells detected in our samples seem to be identical with the recently described CD16low granulocytic subset (37-39). Indeed, it is known for some years that granulocytes reduce their CD16 surface expression during activation (40, 41). Thus, the reduced frequency of CD16low TLR5high expressing cells in nTreg-treated patients as compared to conventionally treated patients indicates reduced inflammation as a direct effect of Treg treatment.
Qian et al showed, that an age-related increase in TLR5+ monocytes is associated with increased inflammatory responses (42), although the authors did not discriminate between CD16+ and CD16− monocytes. Increased peripheral and colonic TLR5 expression has been reported for samples from patients with inflammatory bowel disease (43, 44) and from patients with ankylosing spondolytis (45). Futhermore, Skert and colleagues detected higher TLR5 expression on T cells but especially monocytes of samples from patients with acute GVHD (46), although the authors did not further discriminate between CD16 expressing and CD16 negative monocytes.
Ziegler-Heitbrock first suggested that the differential expression of CD16 could define at least two separate subsets of monocytes with distinct properties (30, 47). CD14+CD16++ monocytes have tissue patrolling function and respond weakly to bacterial TLR ligands (31). Smeekens et al compared the Th17 promoting capacities of CD14++CD16− and CD14+CD16++ monocytes (48). Although both monocyte subsets were able to phagocytose and kill Candida albicans, only CD14++CD16− induced a potent Th17 response. Rommeley et al reported an association between increased numbers of reconstituted CD16 expressing monocytes and reduced risk to develop GVHD (49). Thus, effective GVHD prophylaxis with Tregs may favor the reconstitution of CD14+CD16++ or CD14++CD16+ monocytes, which express less TLR5 and have a more tissue patrolling and repair function in contrast to CD14++CD16− monocytes, which due to their increased TLR and CD14 expression are ready to respond to inflammatory signals such as endogenous and exogenous TLR ligands. This may lead to a reduced risk of GVHD development.
Because UCB nTreg-treated patients without GVHD predominantly were treated with sirolimus, instead of CsA as used for non-nTreg-treated patients and in nTreg-treated patients with GVHD, we cannot discern whether some of the discriminatory findings such as stably decreased peripheral TLR5 expression were related to the use of sirolimus, nTregs or both in combination.
Nonetheless, these findings indicate that UCB transplant patients receiving nTregs combined with sirolimus have a robust tolerance signature that mirrors tolerant patients that have received solid organs. Notably, sirolimus has been reported to favor the development and induction of Tregs, in contrast to CsA (50). Sirolimus, and not CsA, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells (22). Moreover, in preclinical models sirolimus but not CsA is compatible with Treg-mediated suppression of GVHD (24, 51). Thus, it is distinctly possible that sirolimus accentuated the tolerance signature of nTreg infusion in the context of UCB transplants by permitting a higher frequency and biological potency of nTregs early post-transplant as compared to CsA or by inducing Tregs from a CD4+25− non-nTreg population infused in the UCB graft. However, of note, in two studies using non-myeloablative conditioning and matched related donors along with the GVHD prophylactic regimen of MMF + sirolimus, there was a high incidence of acute GVHD causing one study to be prematurely stopped due to excessive acute GVHD (C. Cutler, personal communication) and the second to be discontinued from combined high acute GVHD incidence with excessive toxicity (52).
Moreover,, our investigations also revealed that CD14++CD16− monocytes of nTreg-treated patients regardless of the development of GVHD showed a lower TLR5 expression per cell early after UCB transfer (Figure 7). Additionally as pointed out earlier a faster B cell reconstitution of predominantly naïve B cells and increased B cell related gene expression was observed in samples from nTreg-treated patient despite developing GVHD, which was not observed for samples from conventionally treated patients developing GVHD (Figures 1, 2 and 3). Future studies in which patients are given Tregs or no Tregs and either sirolimus or CsA with MMF will be required to resolve this issue.
Our data reveal similarities between tolerant solid organ and nTreg and predominantly sirolimus treated UCB transplant patients without GVHD, which are characterized by low peripheral TLR5 expression and an altered balance of potentially non-harmful e.g. CD14+CD16++TLR5low and harmful e.g. CD14++CD16−TLR5high leukocyte subpopulations. This may be a direct effect of Tregs on the differentiation of CD16 expressing monocytes. In future investigations it will be important to unravel the communication between Tregs and monocyte subpopulations in more detail in order to utilize that mode of action therapeutically. However, our results set an essential diagnostic basis for validation in further studies, particularly when considering safe drug weaning and Treg treatment trials in solid organ and stem cell transplantation.
Supplementary Material
Acknowledgements and funding sources
Supported in part by DFG grants SFB650 and TR52 to B.S., NIH grants R01 HL56067, AI 34495 (B.R.B.), P01 AI056299 (B.R.B.), NCI P01 CA067493 (B.R.B., J.E.W., J.S.M.) and NHLBI N01HB037164 (J.E.W., J.S.M.), Children’s Cancer Research Fund (BRB, KJL, JEW, JMC, JSM, MRV), Leukemia and Lymphoma Society Scholar in Clinical Research Award # 2417-11(C.G.B.) and the Masonic Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure The authors of this manuscript have no conflicts of interest to disclosure as described by the Biology of Blood and Marrow Transplantation.
References
- 1.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. The lancet oncology. 2011;12(13):1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. The lancet oncology. 2010;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–322. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renard C, Barlogis V, Mialou V, Galambrun C, Bernoux D, Goutagny MP, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. British journal of haematology. 2011;152(3):322–330. doi: 10.1111/j.1365-2141.2010.08409.x. [DOI] [PubMed] [Google Scholar]
- 6.Buhlmann L, Buser AS, Cantoni N, Gerull S, Tichelli A, Gratwohl A, et al. Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone marrow transplantation. 2011;46(10):1357–1362. doi: 10.1038/bmt.2010.306. [DOI] [PubMed] [Google Scholar]
- 7.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 8.Schlieer U, Streitz M, Sawitzki B. Tregs: application for solid-organ transplantation. Current opinion in organ transplantation. 2012;17(1):34–41. doi: 10.1097/MOT.0b013e32834ee69f. [DOI] [PubMed] [Google Scholar]
- 9.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. The Journal of clinical investigation. 2010;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebuhr I, Keeren K, Vogt K, Hoflich C, Appelt C, Schlieer U, et al. Differential expression and function of alpha-mannosidase I in stimulated naive and memory CD4+ T cells. Journal of immunotherapy (Hagerstown, Md : 1997) 2011;34(5):428–437. doi: 10.1097/CJI.0b013e31821dcf23. [DOI] [PubMed] [Google Scholar]
- 11.Pekarsky Y, Palamarchuk A, Maximov V, Efanov A, Nazaryan N, Santanam U, et al. Tcl1 functions as a transcriptional regulator and is directly involved in the pathogenesis of CLL. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19643–19648. doi: 10.1073/pnas.0810965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrizi SJ, Niiro H, Masui M, Yoshimoto G, Iino T, Kikushige Y, et al. T cell leukemia/lymphoma 1 and galectin-1 regulate survival/cell death pathways in human naive and IgM+ memory B cells through altering balances in Bcl-2 family proteins. Journal of immunology (Baltimore, Md : 1950) 2009;182(3):1490–1499. doi: 10.4049/jimmunol.182.3.1490. [DOI] [PubMed] [Google Scholar]
- 13.Ehrhardt GR, Cooper MD. Immunoregulatory roles for fc receptor-like molecules. Current topics in microbiology and immunology. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhardt GR, Leu CM, Zhang S, Aksu G, Jackson T, Haga C, et al. Fc receptor-like proteins (FCRL): immunomodulators of B cell function. Advances in experimental medicine and biology. 2007;596:155–162. doi: 10.1007/0-387-46530-8_14. [DOI] [PubMed] [Google Scholar]
- 15.Arjomand J, Cole S, Evans CJ. Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. Journal of neuroimmunology. 2002;130(1-2):100–108. doi: 10.1016/s0165-5728(02)00217-5. [DOI] [PubMed] [Google Scholar]
- 16.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature immunology. 2008;9(7):769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 17.Shworak NW, Kobayashi T, de Agostini A, Smits NC. Anticoagulant heparan sulfate to not clot--or not? Progress in molecular biology and translational science. 2010;93:153–178. doi: 10.1016/S1877-1173(10)93008-1. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Cruz-Munoz ME, Zhong MC, Chen R, Latour S, Veillette A. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nature immunology. 2009;10(9):973–980. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Calpe S, Westcott J, Castro W, Ma C, Engel P, et al. Cutting edge: The adapters EAT-2A and -2B are positive regulators of CD244- and CD84-dependent NK cell functions in the C57BL/6 mouse. Journal of immunology (Baltimore, Md : 1950) 2010;185(10):5683–5687. doi: 10.4049/jimmunol.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staiano RI, Granata F, Secondo A, Petraroli A, Loffredo S, Frattini A, et al. Expression and function of Na+/Ca2+ exchangers 1 and 3 in human macrophages and monocytes. European journal of immunology. 2009;39(5):1405–1418. doi: 10.1002/eji.200838792. [DOI] [PubMed] [Google Scholar]
- 21.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. The Journal of clinical investigation. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107(3):1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 23.Lim DG, Joe IY, Park YH, Chang SH, Wee YM, Han DJ, et al. Effect of immunosuppressants on the expansion and function of naturally occurring regulatory T cells. Transplant immunology. 2007;18(2):94–100. doi: 10.1016/j.trim.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(22):10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawitzki B, Bushell A, Steger U, Jones N, Risch K, Siepert A, et al. Identification of gene markers for the prediction of allograft rejection or permanent acceptance. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(5):1091–1102. doi: 10.1111/j.1600-6143.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 27.Grogan BM, Tabellini L, Storer B, Bumgarner TE, Astigarraga CC, Flowers ME, et al. Activation and expansion of CD8(+) T effector cells in patients with chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(8):1121–1132. doi: 10.1016/j.bbmt.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lioznov M, El-Cheikh J, Jr., Hoffmann F, Hildebrandt Y, Ayuk F, Wolschke C, et al. Lenalidomide as salvage therapy after allo-SCT for multiple myeloma is effective and leads to an increase of activated NK (NKp44(+)) and T (HLA-DR(+)) cells. Bone marrow transplantation. 2010;45(2):349–353. doi: 10.1038/bmt.2009.155. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Bashey A, Zhong R, Corringham S, Messer K, Pu M, et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(5):682–692. doi: 10.1016/j.bbmt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunology today. 1996;17(9):424–428. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 31.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SJ, Won JH. B cell homeostasis and the development of chronic graft-versus-host disease: implications for B cell-depleting therapy. Leukemia & lymphoma. 2012;53(1):19–25. doi: 10.3109/10428194.2011.603448. [DOI] [PubMed] [Google Scholar]
- 33.Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(1 Suppl):S116–124. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117(7):2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vukicevic M, Chalandon Y, Helg C, Matthes T, Dantin C, Huard B, et al. CD56bright NK cells after hematopoietic stem cell transplantation are activated mature NK cells that expand in patients with low numbers of T cells. European journal of immunology. 2010;40(11):3246–3254. doi: 10.1002/eji.200940016. [DOI] [PubMed] [Google Scholar]
- 37.Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, et al. CD15+/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33(1):121–129. doi: 10.1007/s13277-011-0254-6. [DOI] [PubMed] [Google Scholar]
- 38.Orr Y, Taylor JM, Bannon PG, Geczy C, Kritharides L. Circulating CD10-/CD16low neutrophils provide a quantitative index of active bone marrow neutrophil release. British journal of haematology. 2005;131(4):508–519. doi: 10.1111/j.1365-2141.2005.05794.x. [DOI] [PubMed] [Google Scholar]
- 39.Riera N, Canalejo K, Aixala M, Rosso M, Gaddi E, Bracco MM, et al. Detection of CD16low neutrophil subpopulations. Cytometry Part B, Clinical cytometry. 2003;51(1):45–46. doi: 10.1002/cyto.b.10004. [DOI] [PubMed] [Google Scholar]
- 40.Bzowska M, Hamczyk M, Skalniak A, Guzik K. Rapid decrease of CD16 (FcgammaRIII) expression on heat-shocked neutrophils and their recognition by macrophages. Journal of biomedicine & biotechnology. 2011;2011:284759. doi: 10.1155/2011/284759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. Journal of immunology (Baltimore, Md : 1950) 1994;153(3):1254–1263. [PubMed] [Google Scholar]
- 42.Qian F, Wang X, Zhang L, Chen S, Piecychna M, Allore H, et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging cell. 2012;11(1):104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. The American journal of gastroenterology. 2011;106(2):329–336. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 44.Sipos F, Galamb O, Wichmann B, Krenacs T, Toth K, Leiszter K, et al. Peripheral blood based discrimination of ulcerative colitis and Crohn’s disease from non-IBD colitis by genome-wide gene expression profiling. Disease markers. 2011;30(1):1–17. doi: 10.3233/DMA-2011-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assassi S, Reveille JD, Arnett FC, Weisman MH, Ward MM, Agarwal SK, et al. Whole-blood gene expression profiling in ankylosing spondylitis shows upregulation of toll-like receptor 4 and 5. The Journal of rheumatology. 2011;38(1):87–98. doi: 10.3899/jrheum.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skert C, Fogli M, Perucca S, Garrafa E, Fiorentini S, Fili C, et al. Profile of Toll-Like Receptors on Peripheral Blood Cells in Relation to Acute Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(2):227–234. doi: 10.1016/j.bbmt.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 47.van de Veerdonk FL, Netea MG. Diversity: a hallmark of monocyte society. Immunity. 2010;33(3):289–291. doi: 10.1016/j.immuni.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Smeekens SP, van de Veerdonk FL, Joosten LA, Jacobs L, Jansen T, Williams DL, et al. The classical CD14 CD16 monocytes, but not the patrolling CD14 CD16 monocytes, promote Th17 responses to Candida albicans. European journal of immunology. 2011;41(10):2915–2924. doi: 10.1002/eji.201141418. [DOI] [PubMed] [Google Scholar]
- 49.Rommeley M, Spies-Weisshart B, Schilling K, Hochhaus A, Sayer HG, Scholl S. Reconstitution and functional analyses of neutrophils and distinct subsets of monocytes after allogeneic stem cell transplantation. Journal of cancer research and clinical oncology. 2011;137(9):1293–1300. doi: 10.1007/s00432-011-0989-x. [DOI] [PubMed] [Google Scholar]
- 50.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 51.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118(8):2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston L, Florek M, Armstrong R, McCune JS, Arai S, Brown J, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone marrow transplantation. 2012;47(4):581–588. doi: 10.1038/bmt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.