Abstract
Balancing bone resorption and formation is the quintessential component for the prevention of osteoporosis. Signals that determine the recruitment, replication, differentiation, function, and apoptosis of osteoblasts and osteoclasts direct bone remodeling and determine whether bone tissue is gained, lost, or balanced. Therefore understanding the signaling pathways involved in the coupling process will help develop further targets for osteoporosis therapy, by blocking bone resorption or enhancing bone formation in a space and time dependent manner. Insulin-like growth factor type 1 (IGF-1) has long been known to play a role in bone strength. It is one of the most abundant substances in the bone matrix, circulates systemically and is secreted locally, and has a direct relationship with bone mineral density. Recent data has helped further our understanding of the direct role of IGF-1 signaling in coupling bone remodeling which will be discussed in this review. The bone marrow microenvironment plays a critical role in the fate of MSCs and HSCs and thus how IGF-1 interacts with other factors in the microenvironment are equally important. While previous clinical trials with IGF-1 administration have been unsuccessful at enhancing bone formation, advances in basic science studies have provided insight into further mechanisms that should be considered for future trials. Additional basic science studies dissecting the regulation and the function of matrix IGF-1 in modeling and remodeling will continue to provide further insight for future directions for anabolic therapies for osteoporosis.
Keywords: IGF-1, bone remodeling, mesenchymal stem cell, TGFβ and Coupling
INTRODUCTION
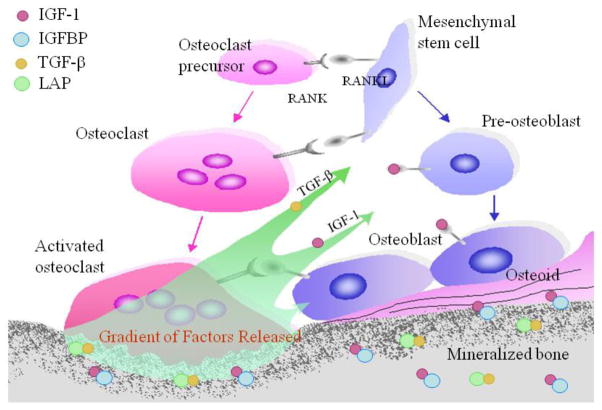
Bone remodeling is a tightly regulated process resulting in the coordinated resorption and formation of skeletal tissue and is an essential process to ensure the integrity of the skeleton. Bone mass peaks in mid to late adolescence, plateaus for several years and then declines over time, increasing skeletal fragility [1–5]. Factors that affect peak bone mass, length of the maintenance phase, and rate of decline all predict osteoporosis risk, a major cause of morbidity and mortality through its association with age-related fractures [6–9]. The hypothesis that factors deposited in the bone matrix act as delayed paracrine agents to couple bone remodeling began over 30 years ago [10–15]. Briefly, latent TGF-β stored in the matrix is released and activated by osteoclasts [16]. Active TGF-β1 then recruits MSCs to the bone-resorptive sites [17]. Similarly, during bone resorption, significant amounts of IGFs are released from storage in either free form or bound to an IGFBP ([18]. IGF-1 produces an osteogenic microenvironment inducing the differentiation of recruited MSCs [19], closely linking bone resorption with formation (Figure 1).
Figure 1. Role of matrix growth factors in coupling bone resorption and formation.
TGF-β1 and IGF-1 are released from the bone matrix in response to osteoclastic bone resorption. TGFβ1 induces migration of MSCs and IGF-1 induces the differentiation and enhances the function of osteoblasts. In response to IGF-1 signaling via IRS-1, osteoblasts enhance expression and secretion of RANKL. RANKL promotes osteoclastogenesis. As a result, new bone formation occurs at bone resorption sites to maintain the microarchitecture and mechanical integrity of the bone in each cycle of bone remodeling, thereby spatially and temporally regulating bone remodeling. IGF-1 – insulin like growth factor type 1; IGFBP – insulin like growth factor binding protein; TGF-β– transforming growth factor-β; LAP – latency associated protein; RANKL - Receptor activator of nuclear factor kappa-B ligand; RANK – receptor for RANKL.
This review will focus specifically on bone matrix IGF-1, both the source and role in coupling remodeling as revealed by clinical and basic science investigations. We also discuss current gaps in our knowledge that may be prohibiting the successful use of IGF-1 as an anabolic agent.
BONE MASS IS TEMPORALLY AND SPATIALY REGULATED
Bone remodeling occurs in basic multicellular units in cycles consisting of three phases: bone resorption by osteoclasts, new bone formation by osteoblasts, and termination of this process. Osteoclasts are multinucleated cells derived from hematopoietic stem cells (HSC), and osteoblasts are mononuclear cells derived from mesenchymal stem cells (MSC) [20]. In order to maintain bone mass, this process must be tightly regulated. The process is coordinated temporally as MSCs are recruited during and after osteoclast resorption. It is also coordinated spatially as MSCs are recruited to the precise site of recent bone resorption for subsequent osteoblast differentiation and bone formation [4;5;21]. Signals that determine the recruitment, replication, differentiation, function, and apoptosis of cells of both lineages direct bone remodeling and are likely regulated by endocrine and paracrine factors, including secretion by local cells and release from the bone matrix, and/or direct cell-cell contact.
CLINICAL OBSERVATIONS
The clinical correlation between IGF-1 and bone mass is well documented. Numerous studies in humans have shown that serum IGF-1 and several of its binding proteins correlate with the pattern of bone mass accrual, maintenance, and loss. Patients with osteoporosis have reduced serum IGF-1 levels, serving as independent predictors for the risk of osteoporosis and fractures [22–37]. Bone marrow IGF-1 concentrations are 40% lower in people with osteoporosis compared to age-matched controls [19]. Skeletal IGF-1 content in human bone declines almost 60% between 20 and 60 years of age [38] and correlates even more strongly with bone volume than serum IGF-1 [39]. Diseases that result in low IGF-1 concentrations also have a higher incidence of low bone mass and osteoporosis compared to the general population and have been comprehensively reviewed by others [25;40;41]. Briefly, anorexia nervosa, a severe eating disorder that leads to progressive malnutrition, is associated with decreased serum IGF-1 levels, hypogonadism, and osteopenia [40]. People with adult onset GH deficiency (aoGHD) may develop a low bone turnover osteoporosis with reduced bone mass and increased fracture risk, correlating with the severity of GHD and circulating levels of IGF-1 [41]. The opposite is also partially true with excess IGF-1 levels, such as seen in acromegaly with overproduction of GH. Acromegaly is associated with increased cortical BMD and increased bone matrix IGF-1, IGF-2, and IGF-BP5 and a high rate of bone turnover, suggesting both osteoblast and osteoclast effects [41;42]. Normalization of GH/IGF-I concentrations is associated with rapid reductions in turnover markers [41].
SOURCE OF IGF-1
As IGF-1 is key to mediating bone modeling and remodeling, many studies have focused on identifying the source of IGF-1. Over the past 15 years the classic somatomedin hypothesis has been modified as tissue specific genetic knockout or overexpression of the IGF-1 ligand has provided further insight to the importance of the source of IGF-1. IGF-1 is synthesized by the liver and circulates acting as an endocrine factor. IGF-1 also acts as a paracrine factor, synthesized by cells such as pre-osteoblasts, mature osteoblasts, osteocytes, and osteoclasts. The contribution of endocrine versus paracrine IGF-1 in bone modeling and remodeling remains an active area of research.
Genetic manipulations of tissue-specific IGF-1 in mouse models have helped delineate the role of endocrine versus paracrine IGF-1. When hepatic Igf-1 expression is prevented (i.e., liver Igf-1-deficient mice), serum Igf-1 concentrations decline by 75% and cortical bone volume is reduced by 26%, with a greater effect on the periosteum in comparison to the endosteum. However, femur length, body weight, and trabecular bone volumes are minimally effected [43]. Transgenic hepatic Igf-1 mice have an earlier acquisition of peak bone mass, but no overall change in bone mass in adults [44]. Similarly, when transgenic hepatic Igf-1 expression is in an Igf-1 null background, although mice are small at birth, the bone phenotype is normalized by adulthood [44;45]. Deletion of Igf-1 in osteoblasts using a collagen 1a2-Cre results in smaller mice, both in weight and length, and decreased mineralization of the skeleton. Reduced osteoblast numbers and activity are suggested, but overall bone volume/tissue volume is unchanged compared to wild type, indicating smaller, compact bones [46]. Overexpression of Igf-1 using an Osteocalcin promoter result in increased rate of bone formation and bone volume/tissue volume at 3 and 6 weeks of age, but the effect is lost by 24 weeks [47]. Overexpression of Igf-1 using a different promoter, Col1a1, showed a similar phenomenon in that by 8 weeks of age, no difference was noted in trabecular bone volume despite a higher mineral apposition rate and greater number of osteoblasts on the bone surface [48]. Deletion of Igf-1 in osteocytes using a Dmp1-Cre results in decreased body size with lower bone mass, but normal BMD, suggesting a low bone turnover state [49]. Deletion of Igf-1 in osteoclast precursors reduces the number of osteoclasts, but the model system used to study this effect precluded further analysis of other bone cells and parameters [50].
While each mouse model has its own limitations, altogether these studies suggest that paracrine IGF-1 has a greater role in body size compared to endocrine IGF-1, but both endocrine and paracrine IGF-1 help regulate bone mass as enhancement of either can make up for the deficit of the opposite. Importantly, overexpression of IGF-1 does not lead to greater BMD in adult mice, but only speeds up the time to attainment of peak bone mass.
REGULATION OF BONE MATRIX IGF-1
Bone matrix IGF-I concentrations have been found to correlate with age-related changes in bone volume more strongly than serum IGF-1 concentrations [19;38]. IGF-1 is one of the most abundant growth factors deposited in the bone matrix [18;39;51–53] and can be released during bone resorption, coupling bone remodeling. However, no studies to date with genetic manipulation of cell-specific IGF-1 expression have evaluated the effect on bone matrix IGF-1 or IGFBP content. Therefore, the source of matrix IGF-1 and mechanisms that regulate its deposition into the skeleton remain unknown, although there is suggestive evidence that both endocrine and paracrine IGF-1 may play a role. Multiple factors have been shown to enhance (PTH, GH, estrogen, T3, BMP2) or suppress (glucocorticoids, PDGF, FGF) IGF-1 transcription in osteoblasts and are associated with higher and lower bone masses, respectively (comprehensively reviewed in [25]). Systemic injection of IGF-1 alone or plus a IGFBP can increase bone mass [19;54–56]. Importantly, for the endocrine IGF-1 effects, only IGF-1 plus IGFBP results in coupled bone formation [19], suggesting that the transport of endocrine IGF-1 may be key to directing the site of IGF-1 action. About 75% of systemic IGF-1 circulates in a 150- to 200-kDa tertiary complex, consisting of IGF-1 + IGFBP + acid labile subunit (ALS). The binding proteins extend the half life of IGF-1 but binding with ALS prohibits transport across the vascular border [57]. About 20–25% of IGF-1 is in a smaller 40- to 50-kDa binary complex (IGF-1 + IGFBP) which can cross the vascular border and may mediate end-target effects [57–59]. Less than 5% of systemic IGF-1 is in the 7.5 kDa free active form. The ratio of binary to tertiary IGF complexes may regulate endocrine IGF-1 effects. Evidence supporting this hypothesis is the observation that IGF-1 plus IGFBP-4, traditionally thought of as inhibitory binding protein, enhances bone mass in mice [54;60;61].
PHYSIOLOGIC FUNCTION OF BONE MATRIX IGF-1
IGFBPs regulate IGF activity, generally favoring storage, while cleavage of the binding protein leads to activation. In general, IGFBP-1, -2, -4, and -6 inhibit and IGFBP-3 and –5 stimulate osteoblast function [62]. While IGFBP-3 is the most abundant binding protein in the circulation, osteoblasts express all six IGFBPs, but IGFBP-4 and -5 most abundantly. All IGFBPs and several IGFBP proteases are found in the skeletal matrix [62]. Transgenic IGFBP4 mice have shorter femoral lengths and decreased bone volumes [61] demonstrating that IGFBP-4 primarily inhibits bone formation by binding and blocking IGF-1 from its receptor [63]. IGFBP-5 stimulates IGF action by either associating with proteins on the cell surface and/or increasing local concentrations of IGFs in the vicinity of the IGF receptors [63]. Preosteoblasts preferentially express IGFBP-2 and -5, whereas mature osteoblasts preferentially express IGFBP-3, -4, and -6 [64], suggesting temporal regulation. Osteoblasts have been found to express multiple specific and non-specific IGFBP proteases: bone morphogenetic protein 1 degrades IGFBP3, pregnancy-associated plasma protein-A degrades IGFBP4, and disintegrin and metalloproteinase domain-containing protein 9 degrade IGFBP-5 [65–68], whereas matrix metalloproteinases can degrade multiple IGFBPs [69]. Osteoclast expression of IGFBP proteases is unknown. Matrix IGF-1 is likely activated by cleavage of IGFBPs by osteoblasts upon resorption of bone matrix by osteoclasts, thereby spatially regulating IGF-1 effects. Mature osteoblasts secrete IGFBPs, which may sequester IGF-1 for deposition into the bone matrix and help terminate the remodeling cycle. If this is true, then the activity of bone matrix IGF-1 released during osteoclast resorption would directly depend on the concentrations/ratio of the IGFBPs in the bone matrix and proteases on target cells.
IGF-1 has multiple roles in promoting bone formation through its actions on cells in the osteoblast lineage. Although migration of MSCs/osteoprogenitors has been suggested by in vitro studies, in vivo studies have not confirmed this role. Specifically, in cell culture, IGF-1 has been shown to enhance expression of genes that promote cell migration, such as chemokine receptor type 4 [70] and activation of Rac and p21-activated protein kinases [71], but how the ligand would increase at the bone resorption site remains unknown. We recently found that MSC migration to the bone remodeling surface was not impaired using an inducible Nestin-CreER::Igf1r knockout mouse model [72]. Direct effects on osteoblastogenesis and osteoblast function have been confirmed in vivo. Using an Osterix-Cre::Igf1r mouse, we found that knockout mice had reduced numbers of mature osteoblasts with lower bone mass and mineral deposition rates than wild type mice, which suggests that IGF-1 released from the bone matrix during bone remodeling stimulates osteoblastic differentiation of MSCs. Further investigation of the cell signaling pathway showed that IGF-1 stimulated osteoblast differentiation was due to activation of mammalian target of rapamycin (mTOR) through the PI3K/Akt pathway [19]. Furthermore, the sensitivity of osteoblast lineage cells to IGF-1 appears to be temporally regulated. Runx2 promotes PI3K-Akt signaling by up-regulating the protein levels of PI3K subunits and Akt, increasing osteoblast differentiation, which can be abrogated by treatment with IGF-1 antibody [73]. The spatial differentiation of osteoblasts at the bone remodeling site is also mediated through regulation of IGF-1 signaling. Osteoclasts express the transmembrane protein, semaphorin 4D (Sema4D). Sema4D binds to its receptor, Plexin-B1 on osteoblasts, inhibiting the RhoA-Rho associated protein kinase (ROCK) pathway, which normally phosphorylates IRS-1, a key factor in the PI3K/Akt/mTOR pathway [74]. These findings suggest that osteoclasts prohibit osteoblast differentiation of cells that come into contact with the osteoclasts, creating a boundary between bone resorption and formation. IGF-1 signaling has also been shown to enhance the function of mature osteoblasts in young mice. Transgenic overexpression of IGF-1 in mice driven by an osteocalcin promoter increased the bone formation rate and trabecular and cortical bone volumes in 3 week-old mice [47].
IGF-1 signaling also influences osteoclast number/function. However, the mechanisms how IGF-1 signaling regulates osteoclast migration, differentiation, and function remain unclear, with the abundance of data being limited to cell culture and indirect measures. IGF-1 has been implicated to mediate morphological changes in osteoclasts that are typical of locomotor cells [75], implying a role in cell migration. IGF-1 null mice show reduced numbers of osteoclasts and reduction of expression markers known to enhance osteoclastogenesis, specifically receptor activator of nuclear factor kappa-B ligand (RANKL), its receptor (RANK) and macrophage colony stimulating factor (M-CSF) [50;76]. However, IGF-1 in culture can only increase the number of mature osteoclasts when co-cultured in the presence of osteoblasts [77–80]. IGF-1 can enhance osteoblast expression and secretion of RANKL in vitro and osteoprotegerin (OPG), a decoy receptor for RANKL, both in vitro and in vivo [81], suggesting osteoblast-dependent effects on osteoclastogenesis. Data are conflicting on the effect of IGF-1 on osteoclast function. Some studies have shown a greater area of resorption pits with IGF-1 administration [77;79], whereas others have shown either no effect or an inhibitory effect [78;80]. It is speculated that the discrepancy may be partially mediated through specific downstream signaling events, particularly through IRS-1 and IRS-2 specific signaling pathways. Global IRS-1−/− mice display a low-turnover osteopenia characterized by decreased number of both osteoblasts and osteoclasts, where osteoblast RANKL expression and secretion are decreased [82]. Oppositely, global IRS-2−/− mice are characterized by high turnover uncoupled bone remodeling with decreased bone formation and increased bone resorption, partly due to the impaired function of osteoblasts but a retained ability to produce RANKL [83]. In vivo studies are needed to clarify these discrepancies.
BONE REMODELING REQUIRES ORCHESTRATION OF SIGNALING NETWORK
To maintain the integrity of the skeleton, crosstalk between signaling pathways is essential. PTH has been found to orchestrate the signaling network of many local osteotropic factors including TGFβs [84], Wnts [85;86], and BMPs [87], coupling bone remodeling. IGF-1 and PTH also work in concert to regulate bone remodeling. The paracrine/autocrine effects of IGF-I are required for the anabolic action of PTH in bone [88;89] through an IRS-1 mediated pathway [90]. Both PTH and IGF-1 regulate cortical bone formation and thickness with synergistic actions. Some effects of the anabolic actions of PTH are mediated by local production of IGF-1 [52;91–95]. IGF-1 also interacts with other hormone signaling pathways. For example, estradiol enhances IGF-1 synthesis in rat bone cells transfected with estrogen receptors [96]. However, in the setting of ovariectomy in rats, IGF-1 treatment results in lower bone volumes compared to untreated ovariectomized mice [97], suggesting a detrimental effect of IGF-1 in the absence of estradiol. Other cells present in the bone marrow, such as T cells may also contribute to changes in the microenvironment. IGF1R signaling inhibits apoptosis of T cells and enhanced T cell activity is seen in acromegaly [98–100], which secrete multiple cytokines that may impact bone cells.
CLINICAL APPLICATIONS
Many clinical trials have explored the use of GH and IGF-1 as anabolic agents. Treatment of specific disease states has resulted in positive results; however, more broad application has been disappointing. Systemic administration of recombinant GH in patients with aoGHD increases bone matrix content of IGF-1 [101], however GH treatment of patients with either normal BMD or osteoporosis have resulted in conflicting results [25]. Females with anorexia nervosa with hypogonadism and osteopenia can only increase BMD with both estrogen and IGF-1 replacement [102]. Elderly women who were treated with a combination of IGFBP-3/IGF-I for 8 weeks following a hip fracture had improved functional recovery and no net loss of femoral bone mass [103]. The use of IGF-1 for the general population in short term trials as measured by increased serum markers of bone turnover has been promising [104–106], particularly one study found that a low dose of IGF-1 preferentially increased serum markers of bone formation over bone resorption [107]. However, a long term trial aimed to treat healthy postmenopausal women without osteoporosis with low dose IGF-1 for 1 year was stopped early as no benefit in BMD, body composition, nor strength was seen [108].
The significant progress that has been made in understanding the role of IGF-1 signaling in bone remodeling has identified potential limitations of these trials. The majority of clinical trials to date have targeted an older population, whereas mouse models with transgenic IGF-1 expression show a loss of benefit in increasing skeletal mass once adulthood is reached [44;45;47;48]. The lack of benefit seen in the older population may be due to the natural down-regulation of the IGF-1 pathway or up-regulation of counter-regulatory mechanisms and account for the relative unresponsiveness. Future trials may need to target younger populations. The duration of therapy required to see an effect is dependent on age and some studies may have been too short based on the age of study participants. For example, the skeletal anabolic effects of GH in the elderly are not seen until 24–36 months, whereas benefits can be seen in children as early as 6 months [109–114]. Mechanical loading has shown to be essential for skeletal response to IGF-1 in mice [115;116]. Therefore, IGF-1 treatment may be more beneficial targeting people who can endure weight-bearing activities. IGF-1 may be more beneficial if administered in conjunction with a binding protein [19;54–56;103] to prolong the half-life and potentially allow for a more precise delivery for the action of IGF-1. IGF-1 enhances bone resorption through increased osteoblast production of RANKL. Combination therapy with an antiresorptive, such as a RANKL inhibitor or bisphosphonate, may improve the outcome. Drug development aimed to modify the expression or activity of IGFBPs may have potential therapeutic implications, similar to the drug developments targeting BMP and Wnt signaling pathways.
Caution is required when considering pursing IGF-1 as a potential anabolic agent. Higher levels of IGF-1 have been associated with an increased risk of specific types of cancer, including prostate and premenopausal breast cancer [117]. Long term data evaluating the safety of GH treatment in childhood, which aims to normalize IGF-1 concentrations, has not shown an overall increase in morbidity or mortality [118–121]. More importantly, the rate of recurrence or development of a second malignancy in survivors of childhood cancer with GH deficiency is the same regardless of whether or not the child is treated with GH [122–124]. Populations that are treated with higher GH doses, such as small for gestational age, and result in supranormal IGF-1 concentrations may be at risk of adverse outcomes [119]. Therefore, extreme care should be used in exclusion criteria and safety monitoring in future trials. Other limitations are the expense of the medication and the route of administration. The only anabolic agent currently FDA approved is PTH which requires the same route of administration. PTH is limited to postmenopausal women and leaves no option for children or premenopausal women with low bone mass/osteoporosis that, interestingly, may be more likely to respond to IGF-1.
KEY APPROACHES FOR FUTURE RESEARCH
Coupling of bone resorption with formation is a critical step for the maintenance of bone mass in the aging skeleton. As high lethality is associated with global knockout models, studies on the role of IGF-1 in this area have progressed by meticulous analysis using the Cre/lox mouse model, which continues to remain an invaluable tool in assessing cell specific effects. As one of the limiting factors in the clinical application of IGF-1 is enhancement of bone resorption, more complete in vivo studies of IGF-1 effects on osteoclastogenesis and osteoclast activity are needed. Mechanisms regulating IGF-1 signaling also need to dissect temporal-spatial effects as the rate and efficiency of bone remodeling changes with age. Careful consideration in the choice of genetic mouse models is needed to comprehensively study temporal effects. Alternative strategies for defining the interaction between IGF-I and bone acquisition have been developed, particularly using congenic models, which may identify novel genes involved in the regulation of IGF-1 signaling.
The orchestration of bone remodeling also requires many additional signaling factors (i.e. RANK/RANKL, TGF-β, Sema4D, plexin B-1, PTH) from osteoblasts, osteoclasts, osteocytes, and skeletal matrix that are also time-, tissue-, and dose-dependent [125]. Understanding the interactions between these pathways is essential to understanding how the bone turnover rate is balanced. As growth factors are ubiquitously expressed, interactions with agents that act specifically on skeletal cells, such as PTH, will provide potential insight into how these agents may be used to manipulate the bone microenvironment.
The age decline in skeletal IGF-1 content suggests that the pool of IGFs may not be sufficiently available for new bone formation as aging ensues and highlights the importance of adequate IGF-1 deposition into the skeleton during modeling and remodeling. Further studies aimed at understanding mechanisms involved in the deposition of IGF-1 into the skeleton are needed.
SUMMARY
Osteoporosis is a major cause of morbidity and mortality through its association with age-related fractures [6–9]. Although most fracture prevention efforts have been directed at inhibition of age-related bone loss, evidence is growing that PBM is an important contributor to bone strength during later life [36;39]. Total skeletal matrix IGF-1 and the amount of IGF-1 released during bone resorption may determine the amount of new bone formation in each cycle of bone remodeling, making it an essential growth factor for the maintenance of skeletal mass. Studies done to date provide insight regarding the role of IGF-1 in the acquisition of PBM and coupled bone remodeling. Successful translation for clinical application still requires answers to several key basic science questions that will help modify clinical designs. Therefore, the continued study of the coordination of bone remodeling at the cellular signaling level, including ways to enhance bone mass, continue to remain a great interest and significance in bone biology and clinical application.
Acknowledgments
This work was supported in part by the grants from the National Institute of Health, including T32DK007751 (JLC) and AR063943 and DK057501 (XC).
Footnotes
Disclosure
The authors declare that they have no conflicts of interests.
Reference List
- 1.Agnusdei D, Gentilella R. GH and IGF-I as therapeutic agents for osteoporosis. J Endocrinol Invest. 2005;28:32–36. [PubMed] [Google Scholar]
- 2.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Schettler AE, Gustafson EM. Osteoporosis prevention starts in adolescence. J Am Acad Nurse Pract. 2004;16:274–282. doi: 10.1111/j.1745-7599.2004.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 5.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 6.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed FA, Ng AC. The pathophysiology of the aging skeleton. Curr Osteoporos Rep. 2010;8:235–240. doi: 10.1007/s11914-010-0035-y. [DOI] [PubMed] [Google Scholar]
- 8.Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–2849. doi: 10.1056/NEJMra040952. [DOI] [PubMed] [Google Scholar]
- 9.Tung S, Iqbal J. Evolution, aging, and osteoporosis. Ann N Y Acad Sci. 2007;1116:499–506. doi: 10.1196/annals.1402.080. [DOI] [PubMed] [Google Scholar]
- 10.Hayden JM, Mohan S, Baylink DJ. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 1995;17:93S–98S. doi: 10.1016/8756-3282(95)00186-h. [DOI] [PubMed] [Google Scholar]
- 11.Oreffo RO, Mundy GR, Seyedin SM, Bonewald LF. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun. 1989;158:817–823. doi: 10.1016/0006-291x(89)92795-2. [DOI] [PubMed] [Google Scholar]
- 12.Mundy GR, Rodan SB, Majeska RJ, DeMartino S, Trimmier C, Martin TJ, Rodan GA. Unidirectional migration of osteosarcoma cells with osteoblast characteristics in response to products of bone resorption. Calcif Tissue Int. 1982;34:542–546. doi: 10.1007/BF02411301. [DOI] [PubMed] [Google Scholar]
- 13.Somerman MJ, Hotchkiss RN, Bowers MR, Termine J. Comparison of fetal and adult human bone: identification of a chemotactic factor in fetal bone. Metab Bone Dis Relat Res. 1983;5:75–79. doi: 10.1016/0221-8747(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 14.Parfitt AM. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int. 1984;36(Suppl 1):S37–S45. doi: 10.1007/BF02406132. [DOI] [PubMed] [Google Scholar]
- 15.Canalis E, McCarthy TL, Centrella M. The role of growth factors in skeletal remodeling. Endocrinol Metab Clin North Am. 1989;18:903–918. [PubMed] [Google Scholar]
- 16.Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van HW, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan S, Jennings JC, Linkhart TA, Baylink DJ. Primary structure of human skeletal growth factor: homology with human insulin-like growth factor-II. Biochim Biophys Acta. 1988;966:44–55. doi: 10.1016/0304-4165(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 19.Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canalis E. The fate of circulating osteoblasts. N Engl J Med. 2005;352:2014–2016. doi: 10.1056/NEJMe058080. [DOI] [PubMed] [Google Scholar]
- 21.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Amin S, Riggs BL, Melton LJ, III, Achenbach SJ, Atkinson EJ, Khosla S. High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res. 2007;22:799–807. doi: 10.1359/jbmr.070306. [DOI] [PubMed] [Google Scholar]
- 23.Canalis E. Skeletal growth factors and aging. J Clin Endocrinol Metab. 1994;78:1009–1010. doi: 10.1210/jcem.78.5.8175952. [DOI] [PubMed] [Google Scholar]
- 24.Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone mineral density in femoral neck is positively correlated to circulating insulin-like growth factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men. Calcif Tissue Int. 2002;70:22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- 25.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 27.Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–4262. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- 28.Mezquita-Raya P, Munoz-Torres M, Alonso G, de Luna JD, Quesada JM, Dorado G, Luque-Recio F, Ruiz-Requena ME, Lopez-Rodriguez F, Escobar-Jimenez F. Susceptibility for postmenopausal osteoporosis: interaction between genetic, hormonal and lifestyle factors. Calcif Tissue Int. 2004;75:373–379. doi: 10.1007/s00223-004-0187-9. [DOI] [PubMed] [Google Scholar]
- 29.Patel MB, Arden NK, Masterson LM, Phillips DI, Swaminathan R, Syddall HE, Byrne CD, Wood PJ, Cooper C, Holt RI. Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF) axis as a determinant of male bone mineral density (BMD) Bone. 2005;37:833–841. doi: 10.1016/j.bone.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Rhee EJ, Oh KW, Lee WY, Kim SW, Oh ES, Baek KH, Kang MI, Park CY, Choi MG, Yoo HJ, Park SW. Age, body mass index, current smoking history, and serum insulin-like growth factor-I levels associated with bone mineral density in middle-aged Korean men. J Bone Miner Metab. 2004;22:392–398. doi: 10.1007/s00774-003-0500-0. [DOI] [PubMed] [Google Scholar]
- 31.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Kanatani M, Yamauchi M, Kaji H, Sugishita T, Baylink DJ, Mohan S, Chihara K, Sugimoto T. Serum levels of insulin-like growth factor (IGF); IGF-binding proteins-3, -4, and -5; and their relationships to bone mineral density and the risk of vertebral fractures in postmenopausal women. Calcif Tissue Int. 2006;78:18–24. doi: 10.1007/s00223-005-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziv E, Hu D. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res Rev. 2011;10:201–204. doi: 10.1016/j.arr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, Broos P, Bouillon R, Baylink DJ. Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res. 1999;14:2150–2158. doi: 10.1359/jbmr.1999.14.12.2150. [DOI] [PubMed] [Google Scholar]
- 35.Kurland ES, Rosen CJ, Cosman F, McMahon D, Chan F, Shane E, Lindsay R, Dempster D, Bilezikian JP. Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1997;82:2799–2805. doi: 10.1210/jcem.82.9.4253. [DOI] [PubMed] [Google Scholar]
- 36.Ohlsson C, Mellstrom D, Carlzon D, Orwoll E, Ljunggren O, Karlsson MK, Vandenput L. Older men with low serum IGF-1 have an increased risk of incident fractures: the MrOS Sweden study. J Bone Miner Res. 2011;26:865–872. doi: 10.1002/jbmr.281. [DOI] [PubMed] [Google Scholar]
- 37.Jehle PM, Schulten K, Schulz W, Jehle DR, Stracke S, Manfras B, Boehm BO, Baylink DJ, Mohan S. Serum levels of insulin-like growth factor (IGF)-I and IGF binding protein (IGFBP)-1 to -6 and their relationship to bone metabolism in osteoporosis patients. Eur J Intern Med. 2003;14:32–38. doi: 10.1016/s0953-6205(02)00183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolas V, Prewett A, Bettica P, Mohan S, Finkelman RD, Baylink DJ, Farley JR. Age-related decreases in insulin-like growth factor-I and transforming growth factor-beta in femoral cortical bone from both men and women: implications for bone loss with aging. J Clin Endocrinol Metab. 1994;78:1011–1016. doi: 10.1210/jcem.78.5.8175953. [DOI] [PubMed] [Google Scholar]
- 39.Seck T, Scheppach B, Scharla S, Diel I, Blum WF, Bismar H, Schmid G, Krempien B, Ziegler R, Pfeilschifter J. Concentration of insulin-like growth factor (IGF)-I and -II in iliac crest bone matrix from pre- and postmenopausal women: relationship to age, menopause, bone turnover, bone volume, and circulating IGFs. J Clin Endocrinol Metab. 1998;83:2331–2337. doi: 10.1210/jcem.83.7.4967. [DOI] [PubMed] [Google Scholar]
- 40.Misra M, Klibanski A. Anorexia nervosa and osteoporosis. Rev Endocr Metab Disord. 2006;7:91–99. doi: 10.1007/s11154-006-9005-1. [DOI] [PubMed] [Google Scholar]
- 41.Ueland T. GH/IGF-I and bone resorption in vivo and in vitro. Eur J Endocrinol. 2005;152:327–332. doi: 10.1530/eje.1.01874. [DOI] [PubMed] [Google Scholar]
- 42.Ueland T, Bollerslev J, Hansen TB, Ebbesen EN, Mosekilde L, Brixen K, Flyvbjerg A, Djoseland O. Increased cortical bone content of insulin-like growth factors in acromegalic patients. J Clin Endocrinol Metab. 1999;84:123–127. doi: 10.1210/jcem.84.1.5384. [DOI] [PubMed] [Google Scholar]
- 43.Yakar S, Rosen CJ, Beamer WG, ckert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elis S, Courtland HW, Wu Y, Rosen CJ, Sun H, Jepsen KJ, Majeska RJ, Yakar S. Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties even in the absence of tissue IGF-1. J Bone Miner Res. 2010;25:1257–1266. doi: 10.1002/jbmr.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105:19378–19383. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148:5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 48.Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:494–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 49.Sheng MH, Zhou XD, Bonewald LF, Baylink DJ, Lau KH. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52:133–144. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21:1350–1358. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canalis E, Pash J, Gabbitas B, Rydziel S, Varghese S. Growth factors regulate the synthesis of insulin-like growth factor-I in bone cell cultures. Endocrinology. 1993;133:33–38. doi: 10.1210/endo.133.1.8319580. [DOI] [PubMed] [Google Scholar]
- 52.Pfeilschifter J, Laukhuf F, Muller-Beckmann B, Blum WF, Pfister T, Ziegler R. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J Clin Invest. 1995;96:767–774. doi: 10.1172/JCI118121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frolik CA, Ellis LF, Williams DC. Isolation and characterization of insulin-like growth factor-II from human bone. Biochem Biophys Res Commun. 1988;151:1011–1018. doi: 10.1016/s0006-291x(88)80466-2. [DOI] [PubMed] [Google Scholar]
- 54.Miyakoshi N, Qin X, Kasukawa Y, Richman C, Srivastava AK, Baylink DJ, Mohan S. Systemic administration of insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) increases bone formation parameters in mice by increasing IGF bioavailability via an IGFBP-4 protease-dependent mechanism. Endocrinology. 2001;142:2641–2648. doi: 10.1210/endo.142.6.8192. [DOI] [PubMed] [Google Scholar]
- 55.Bagi CM, DeLeon E, Brommage R, Rosen D, Sommer A. Treatment of ovariectomized rats with the complex of rhIGF-I/IGFBP-3 increases cortical and cancellous bone mass and improves structure in the femoral neck. Calcif Tissue Int. 1995;57:40–46. doi: 10.1007/BF00298995. [DOI] [PubMed] [Google Scholar]
- 56.Bauss F, Lang K, Dony C, Kling L. The complex of recombinant human insulin-like growth factor-I (rhIGF-I) and its binding protein-5 (IGFBP-5) induces local bone formation in murine calvariae and in rat cortical bone after local or systemic administration. Growth Horm IGF Res. 2001;11:1–9. doi: 10.1054/ghir.2000.0181. [DOI] [PubMed] [Google Scholar]
- 57.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 58.Binoux M, Hossenlopp P. Insulin-like growth factor (IGF) and IGF-binding proteins: comparison of human serum and lymph. J Clin Endocrinol Metab. 1988;67:509–514. doi: 10.1210/jcem-67-3-509. [DOI] [PubMed] [Google Scholar]
- 59.Bar RS, Boes M, Dake BL, Sandra A, Bayne M, Cascieri M, Booth BA. Tissue localization of perfused endothelial cell IGF binding protein is markedly altered by association with IGF-I. Endocrinology. 1990;127:3243–3245. doi: 10.1210/endo-127-6-3243. [DOI] [PubMed] [Google Scholar]
- 60.Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–5728. doi: 10.1210/endo.140.12.7175. [DOI] [PubMed] [Google Scholar]
- 61.Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL. Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res. 2003;18:836–843. doi: 10.1359/jbmr.2003.18.5.836. [DOI] [PubMed] [Google Scholar]
- 62.Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol. 2005;20:261–268. doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 64.Birnbaum RS, Wiren KM. Changes in insulin-like growth factor-binding protein expression and secretion during the proliferation, differentiation, and mineralization of primary cultures of rat osteoblasts. Endocrinology. 1994;135:223–230. doi: 10.1210/endo.135.1.8013356. [DOI] [PubMed] [Google Scholar]
- 65.Kim B, Huang G, Ho WB, Greenspan DS. Bone morphogenetic protein-1 processes insulin-like growth factor-binding protein 3. J Biol Chem. 2011;286:29014–29025. doi: 10.1074/jbc.M111.252585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, III, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 68.Qin X, Sexton C, Byun D, Strong DD, Baylink DJ, Mohan S. Differential regulation of pregnancy associated plasma protein (PAPP)-A during pregnancy in human and mouse. Growth Horm IGF Res. 2002;12:359–366. doi: 10.1016/s1096-6374(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 69.Thrailkill KM, Quarles LD, Nagase H, Suzuki K, Serra DM, Fowlkes JL. Characterization of insulin-like growth factor-binding protein 5-degrading proteases produced throughout murine osteoblast differentiation. Endocrinology. 1995;136:3527–3533. doi: 10.1210/endo.136.8.7543045. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 71.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 72.Crane JL, Zhao L, Frye JS, Xian L, Qiu T, Cao X. IGF-1 signaling is essential for differentiation of mesenchymal stem cells for peak bone mass. Bone Research. 2013;2:186–194. doi: 10.4248/BR201302007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, Ogita K, Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 75.Fiorelli G, Formigli L, Zecchi OS, Gori F, Falchetti A, Morelli A, Tanini A, Benvenuti S, Brandi ML. Characterization and function of the receptor for IGF-I in human preosteoclastic cells. Bone. 1996;18:269–276. doi: 10.1016/8756-3282(95)00485-8. [DOI] [PubMed] [Google Scholar]
- 76.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 77.Hill PA, Reynolds JJ, Meikle MC. Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology. 1995;136:124–131. doi: 10.1210/endo.136.1.7828521. [DOI] [PubMed] [Google Scholar]
- 78.Jonsson KB, Wiberg K, Ljunghall S, Ljunggren O. Insulin-like growth factor I does not stimulate bone resorption in cultured neonatal mouse calvarial bones. Calcif Tissue Int. 1996;59:366–370. doi: 10.1007/s002239900141. [DOI] [PubMed] [Google Scholar]
- 79.Mochizuki H, Hakeda Y, Wakatsuki N, Usui N, Akashi S, Sato T, Tanaka K, Kumegawa M. Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology. 1992;131:1075–1080. doi: 10.1210/endo.131.3.1505451. [DOI] [PubMed] [Google Scholar]
- 80.Slootweg MC, Most WW, van BE, Schot LP, Papapoulos SE, Lowik CW. Osteoclast formation together with interleukin-6 production in mouse long bones is increased by insulin-like growth factor-I. J Endocrinol. 1992;132:433–438. doi: 10.1677/joe.0.1320433. [DOI] [PubMed] [Google Scholar]
- 81.Rubin J, ckert-Bicknell CL, Zhu L, Fan X, Murphy TC, Nanes MS, Marcus R, Holloway L, Beamer WG, Rosen CJ. IGF-I regulates osteoprotegerin (OPG) and receptor activator of nuclear factor-kappaB ligand in vitro and OPG in vivo. J Clin Endocrinol Metab. 2002;87:4273–4279. doi: 10.1210/jc.2002-020656. [DOI] [PubMed] [Google Scholar]
- 82.Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000;105:935–943. doi: 10.1172/JCI9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akune T, Ogata N, Hoshi K, Kubota N, Terauchi Y, Tobe K, Takagi H, Azuma Y, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J Cell Biol. 2002;159:147–156. doi: 10.1083/jcb.200204046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol. 2010;12:224–234. doi: 10.1038/ncb2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wan M, Li J, Herbst K, Zhang J, Yu B, Wu X, Qiu T, Lei W, Lindvall C, Williams BO, Ma H, Zhang F, Cao X. LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Galpha(s) Sci Signal. 2011;4:ra15. doi: 10.1126/scisignal.2001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, Wan M, Cao X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012;27:2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001;142:4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146:2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]
- 91.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 92.Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989;83:60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elis S, Courtland HW, Wu Y, Fritton JC, Sun H, Rosen CJ, Yakar S. Elevated serum IGF-1 levels synergize PTH action on the skeleton only when the tissue IGF-1 axis is intact. J Bone Miner Res. 2010;25:2051–2058. doi: 10.1002/jbmr.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lombardi G, Di SC, Vuolo L, Guerra E, Scarano E, Colao A. Role of IGF-I on PTH effects on bone. J Endocrinol Invest. 2010;33:22–26. [PubMed] [Google Scholar]
- 95.Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone. 1995;16:357–365. doi: 10.1016/8756-3282(94)00051-4. [DOI] [PubMed] [Google Scholar]
- 96.Ernst M, Rodan GA. Estradiol regulation of insulin-like growth factor-I expression in osteoblastic cells: evidence for transcriptional control. Mol Endocrinol. 1991;5:1081–1089. doi: 10.1210/mend-5-8-1081. [DOI] [PubMed] [Google Scholar]
- 97.Ibbotson KJ, Orcutt CM, D’Souza SM, Paddock CL, Arthur JA, Jankowsky ML, Boyce RW. Contrasting effects of parathyroid hormone and insulin-like growth factor I in an aged ovariectomized rat model of postmenopausal osteoporosis. J Bone Miner Res. 1992;7:425–432. doi: 10.1002/jbmr.5650070410. [DOI] [PubMed] [Google Scholar]
- 98.Brocardo MG, Schillaci R, Galeano A, Radrizzani M, White V, Guerrico AG, Santa-Coloma TA, Roldan A. Early effects of insulin-like growth factor-1 in activated human T lymphocytes. J Leukoc Biol. 2001;70:297–305. [PubMed] [Google Scholar]
- 99.Walsh PT, O’Connor R. The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol. 2000;30:1010–1018. doi: 10.1002/(SICI)1521-4141(200004)30:4<1010::AID-IMMU1010>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 100.Colao A, Ferone D, Marzullo P, Panza N, Pivonello R, Orio F, Jr, Grande G, Bevilacqua N, Lombardi G. Lymphocyte subset pattern in acromegaly. J Endocrinol Invest. 2002;25:125–128. doi: 10.1007/BF03343975. [DOI] [PubMed] [Google Scholar]
- 101.Ueland T, Bollerslev J, Flyvbjerg A, Hansen TB, Vahl N, Mosekilde L. Effects of 12 months of GH treatment on cortical and trabecular bone content of IGFs and OPG in adults with acquired GH deficiency: a double-blind, randomized, placebo-controlled study. J Clin Endocrinol Metab. 2002;87:2760–2763. doi: 10.1210/jcem.87.6.8549. [DOI] [PubMed] [Google Scholar]
- 102.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 103.Boonen S, Rosen C, Bouillon R, Sommer A, McKay M, Rosen D, Adams S, Broos P, Lenaerts J, Raus J, Vanderschueren D, Geusens P. Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: a double-blind, placebo-controlled pilot study. J Clin Endocrinol Metab. 2002;87:1593–1599. doi: 10.1210/jcem.87.4.8426. [DOI] [PubMed] [Google Scholar]
- 104.Ebeling PR, Jones JD, O’Fallon WM, Janes CH, Riggs BL. Short-term effects of recombinant human insulin-like growth factor I on bone turnover in normal women. J Clin Endocrinol Metab. 1993;77:1384–1387. doi: 10.1210/jcem.77.5.8077337. [DOI] [PubMed] [Google Scholar]
- 105.Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995;96:900–906. doi: 10.1172/JCI118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johansson AG, Lindh E, Ljunghall S. Insulin-like growth factor I stimulates bone turnover in osteoporosis. Lancet. 1992;339:1619. doi: 10.1016/0140-6736(92)91889-g. [DOI] [PubMed] [Google Scholar]
- 107.Ghiron LJ, Thompson JL, Holloway L, Hintz RL, Butterfield GE, Hoffman AR, Marcus R. Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res. 1995;10:1844–1852. doi: 10.1002/jbmr.5650101203. [DOI] [PubMed] [Google Scholar]
- 108.Friedlander AL, Butterfield GE, Moynihan S, Grillo J, Pollack M, Holloway L, Friedman L, Yesavage J, Matthias D, Lee S, Marcus R, Hoffman AR. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- 109.Janssen YJ, Hamdy NA, Frolich M, Roelfsema F. Skeletal effects of two years of treatment with low physiological doses of recombinant human growth hormone (GH) in patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1998;83:2143–2148. doi: 10.1210/jcem.83.6.4851. [DOI] [PubMed] [Google Scholar]
- 110.Johannsson G, Rosen T, Bosaeus I, Sjostrom L, Bengtsson BA. Two years of growth hormone (GH) treatment increases bone mineral content and density in hypopituitary patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1996;81:2865–2873. doi: 10.1210/jcem.81.8.8768843. [DOI] [PubMed] [Google Scholar]
- 111.Baum HB, Biller BM, Finkelstein JS, Cannistraro KB, Oppenhein DS, Schoenfeld DA, Michel TH, Wittink H, Klibanski A. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med. 1996;125:883–890. doi: 10.7326/0003-4819-125-11-199612010-00003. [DOI] [PubMed] [Google Scholar]
- 112.Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 113.Rahim A, Holmes SJ, Adams JE, Shalet SM. Long-term change in the bone mineral density of adults with adult onset growth hormone (GH) deficiency in response to short or long-term GH replacement therapy. Clin Endocrinol (Oxf) 1998;48:463–469. doi: 10.1046/j.1365-2265.1998.00465.x. [DOI] [PubMed] [Google Scholar]
- 114.Drake WM, Carroll PV, Maher KT, Metcalfe KA, Camacho-Hubner C, Shaw NJ, Dunger DB, Cheetham TD, Savage MO, Monson JP. The effect of cessation of growth hormone (GH) therapy on bone mineral accretion in GH-deficient adolescents at the completion of linear growth. J Clin Endocrinol Metab. 2003;88:1658–1663. doi: 10.1210/jc.2002-021541. [DOI] [PubMed] [Google Scholar]
- 115.Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003;32:669–680. doi: 10.1016/s8756-3282(03)00088-7. [DOI] [PubMed] [Google Scholar]
- 116.Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004;19:436–446. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 118.Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95:167–177. doi: 10.1210/jc.2009-0178. [DOI] [PubMed] [Google Scholar]
- 119.Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, Coste J. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97:416–425. doi: 10.1210/jc.2011-1995. [DOI] [PubMed] [Google Scholar]
- 120.Savendahl L, Maes M, bertsson-Wikland K, Borgstrom B, Carel JC, Henrard S, Speybroeck N, Thomas M, Zandwijken G, Hokken-Koelega A. Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands, and Sweden: preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab. 2012;97:E213–E217. doi: 10.1210/jc.2011-2882. [DOI] [PubMed] [Google Scholar]
- 121.Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database) J Pediatr. 2010;157:265–270. doi: 10.1016/j.jpeds.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 122.Mackenzie S, Craven T, Gattamaneni HR, Swindell R, Shalet SM, Brabant G. Long-term safety of growth hormone replacement after CNS irradiation. J Clin Endocrinol Metab. 2011;96:2756–2761. doi: 10.1210/jc.2011-0112. [DOI] [PubMed] [Google Scholar]
- 123.Ergun-Longmire B, Mertens AC, Mitby P, Qin J, Heller G, Shi W, Yasui Y, Robison LL, Sklar CA. Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. J Clin Endocrinol Metab. 2006;91:3494–3498. doi: 10.1210/jc.2006-0656. [DOI] [PubMed] [Google Scholar]
- 124.Sklar CA, Mertens AC, Mitby P, Occhiogrosso G, Qin J, Heller G, Yasui Y, Robison LL. Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2002;87:3136–3141. doi: 10.1210/jcem.87.7.8606. [DOI] [PubMed] [Google Scholar]
- 125.Cao X. Targeting osteoclast-osteoblast communication. Nat Med. 2011;17:1344–1346. doi: 10.1038/nm.2499. [DOI] [PubMed] [Google Scholar]