Fig. 3.
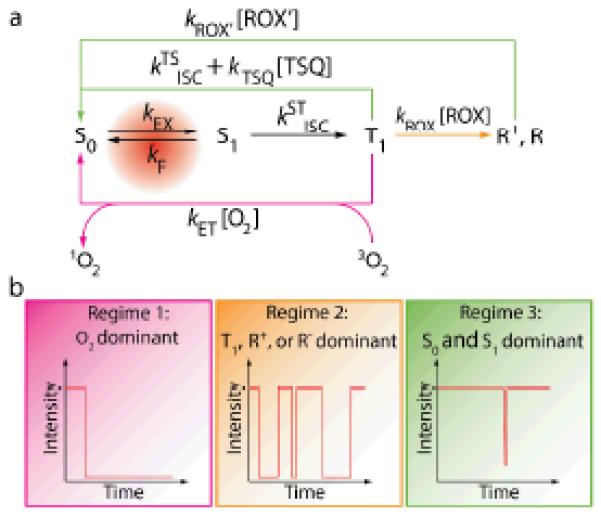
(a) A framework for understanding the nature of fluorophore instabilities. (b) Kinetic regimes that lead to different behaviors of a fluorophore. TSQ: triplet state quencher; ROX and ROX’: reducing or oxidizing agents. texp: the exposure time for each frame of the measurement. Regime 1 occurs when
ket[O2] >> ktsisc+ktsq[TSQ], krox[ROX],
and the fluorophore photobleaches quickly. Regime 2 occurs when
krox[ROX] >> ket[O2], ktsisc +ktsq[TSQ];
krox’[ROX’] ≤ 1/ texp,
and the fluorophore blinks frequently. Regime 3 occurs when
ktsisc+ ktsq[TSQ] >> ket[O2], krox[ROX];
or krox[ROX] >> ket[O2], ktsisc +ktsq[TSQ];
krox’[ROX’] >> 1/ texp,
and the fluorophore lasts long and rarely blinks.
