Fig. 4.
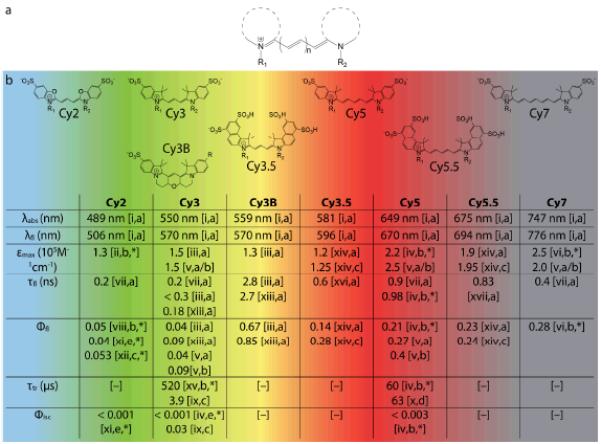
(a) Generic structure of cyanine fluorophores. (b) Structures of commercially available cyanine fluorophores along with important spectroscopic properties. Note that the values cited here are for free dyes in solution and may change significantly upon conjugation to biomolecules. λabs, λem – wavelengths of absorption, emission maximum; εmax – extinction coefficient; τfl, τtr – lifetimes of fluorescence and triplet state; Φfl, Φisc – quantum yields of fluorescence and intersystem crossing. The R groups represent the various linkers available for bioconjugation of the fluorophores.
Source: [i] Dempsey et al. 2011;98 [ii] Kassab 2002;65 [iii] Cooper et al. 2004;100 [iv] Chibisov et al. 1996;70 [v] Mujumdar et al. 1993;99 [vi] Rurack and Spieles 2011;101 [vii] unpublished data; [viii] Ponterini and Caselli 1992;102 [ix] Jia et al. 2007;75 [x] Zheng et al. 2012;76 [xi] Chibisov 1977;103 [xii] Roth and Craig 1974;104 [xiii] Sanborn et al. 2007;105 [xiv] Mujumdar et al. 1996;106 [xv] Chibisov et al. 1995;107 [xvi] Gu et al. 2013;108 [xvii] Buschmann et al. 2003.67 Solvent: [a] Water; [b] Ethanol; [c] Methanol; [d] Acetonitrile; [e] Butanol. [*] non-sulfonated form; [–] no data available.
