Figure 3. Topological constraints necessitate conformational switching.
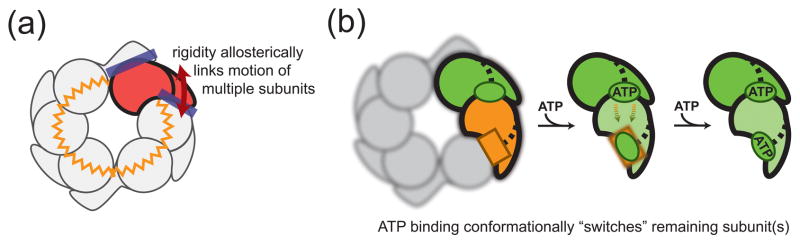
(A) The rigid interfaces between neighboring subunits as well as the closed-ring topology cause conformational changes of a single subunit to also change the conformation of other subunits. The conformational coupling of subunits throughout the hexamer is indicated by a zigzag circle. (B) ATP binding to a subunit results in a hinge-like motion of the small domain about its N-linker (see Fig. 2C). Other subunits within the ring are forced to switch their conformation (orange-colored subunit changes green) and, equivalently, change their nucleotide binding affinity (square binding site changes its shape). The spatial order of conformational switching and the detailed conformation of switched subunits remain unclear. This illustration depicts the simplest case involving adjacent subunits, with the remainder of the ring blurred from view.
