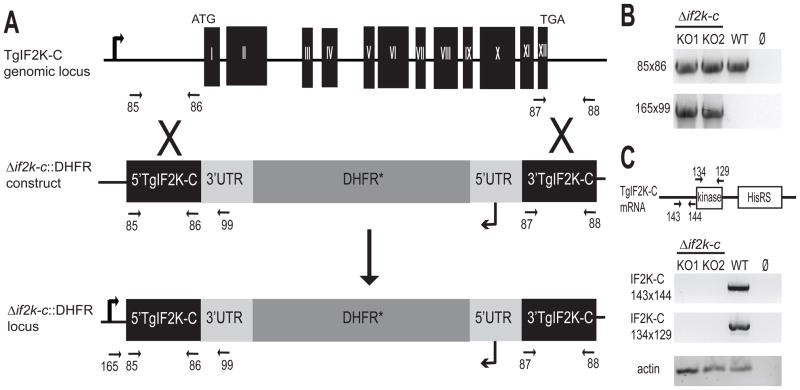
Fig. 3.
Generation of Toxoplasma gondii (Tg)IF2K-C knockout (KO) parasites. (A) Genomic fragments upstream and downstream of the open reading frame were amplified by PCR using genomic DNA and oligonucleotide primers #85 and #86, and #87 and #88 (indicated by arrows), respectively. The resulting DNA fragments flank a dihydrofolate reductase (DHFR*) selection marker to comprise the knockout plasmid Δif2k-c::DHFR. The knockout construct was used to replace the complete TgIF2K-C genomic locus via homologous recombination in RHΔku80 strain (referred to as wild-type or WT). (B) The replacement of the TgIF2K-C locus in two independent clones (KO1, KO2) was analyzed by PCR using oligonucleotides complementary to DNA sequences upstream of the replacement site (oligonucleotide #165) and in the 3′-untranslated region (UTR) of the DHFR* minigene (oligonucleotide #99). WT genomic DNA was included as a positive control for amplification. (C) Reverse transcriptase (RT)-PCR using oligonucleotides that amplify a ~1.0 kb fragment at the 5′ end (oligonucleotides #143 and #144) and the fragment encoding the protein kinase domain (oligonucleotides #132 and #129) were carried out to verify the absence of TgIF2K-C mRNA in the knockout clones. As a control for the mRNA preparation, a portion of Toxoplasma actin mRNA was amplified by RT-PCR. To exclude contaminating genomic DNA, the template was omitted from one PCR sample (Ø).