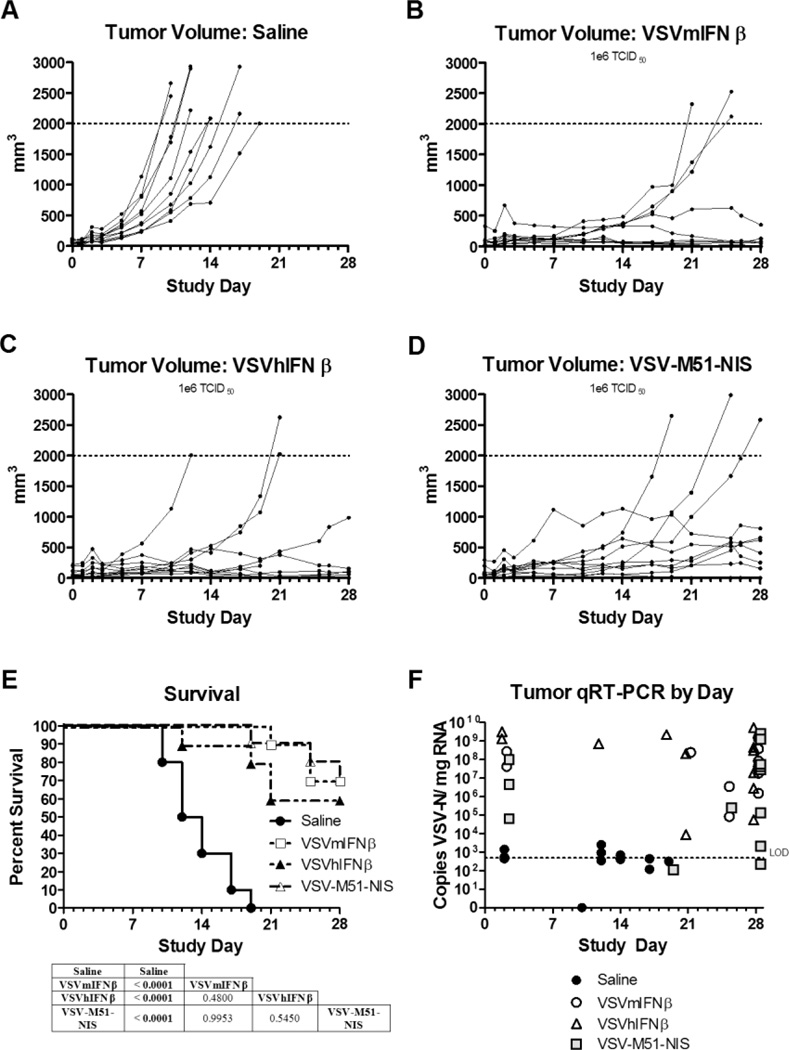
Figure 5.
Treatment effects of IV VSV on endometrial xenografts derived from the AN3CA cell line. Subcutaneous xenografts were established in the right flank of athymic nude mice. Once tumors formed, the mice were assigned randomly to a single IV injection of saline or different VSV variants (1×106 TCID50). A–D, Individual tumor volumes for the duration of the study for each study group: saline (A), VSV-mIFNβ (B), VSV-hIFNβ (C), and VSV-M51-NIS (D). E, Survival of each group was plotted on a Kaplan-Meier survival curve. P values were determined by the log-rank (Mantel-Cox) method and shown as tables underneath the survival curve of each tumor model. P<.05 was considered significant. F, A qRT-PCR was performed to quantify VSV-N mRNA in tumors on mice necropsied after meeting euthanasia criteria during the efficacy portion of the study or necropsied at the end of the study except for the day 2 samples. Two or 3 mice from each group were euthanized 2 days after treatment to determine the acute effects of viral therapy and spread of virus (safety study). The limit of reliable detection (LOD) for the assay was 500 copies/µg (dotted horizontal line on graphs). Copy numbers less than 500 were interpreted as suspect results. Ten mice were included in the efficacy study and 2 or 3 mice in the safety study.