Table 3.
Oxidation of 2,2′-bistryptamine derivatives.
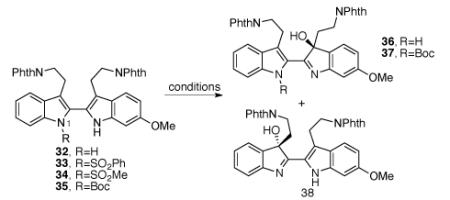
| Entry | substrate | condition | yield (product) | % ee (product) |
|---|---|---|---|---|
| 1 | 32 | 19, CH2Cl2, 0 °C | 91% (36:38 = 1:1)a | - |
| 2 | 32 | air, neat, 23 °C, 12 d | 27% (36:38 = 1.5:1)a,b | - |
| 3 | 33 | 19, CH2Cl2, 23→50 °C | No reaction | - |
| 4 | 34 | 19, CH2Cl2, 23 °C | No reaction | - |
| 5 | 34 | mCPBA, CH2Cl2, 23 °C, 3 h | decomposition | - |
| 6 | 35 | 19, CH2Cl2, 23→65 °C | No reaction | - |
| 7 | 35 | DMDO, acetone, 23 °C, 11 h | 32% (37) | - |
| 8 | 35 | Shi epoxidationc | No reaction | - |
| 9 | 35 | Sharpless asymmetric dihydroxylationd |
No reaction | - |
| 10 | 32 | Sharpless asymmetric dihydroxylationd |
39% (36:38 = 1.7:1)a | <3 (36), <3 (38) |
| 11 | 32 |
40 (10 mol%),DMAP, H2O2, DIC, CHCl3, 0 °C, 24.5 h |
49% (36:38 = 1.3:1)a | 20 (36), <5 (38) |
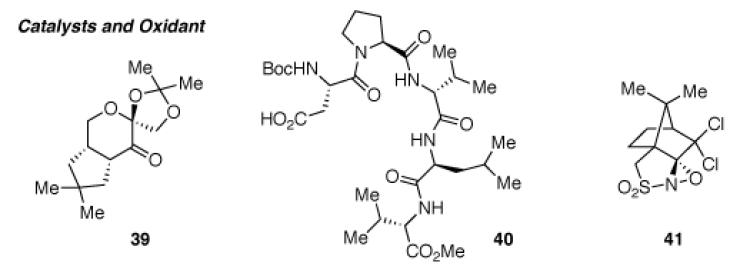
| 12 | 32 | 41, CH2Cl2, −30→23 °C | 95% (36:38 = 2.2:1)a | 96 (36), 96 (38) |
|
| ||||
| ||||
The ratio of 36 to 38 was determined by 1H NMR.
65% of unreacted starting material 33 was isolated.
Shi catalyst (39), Na2B2O7•H2O, n-Bu4NHSO4, oxone, K2CO3, EDTA, H2O, DMM, MeCN.
AD-mix- α , methanesulfonamide, t-BuOH, H2O.
