Figure 1. Stalled ACPs bound to LpxD.
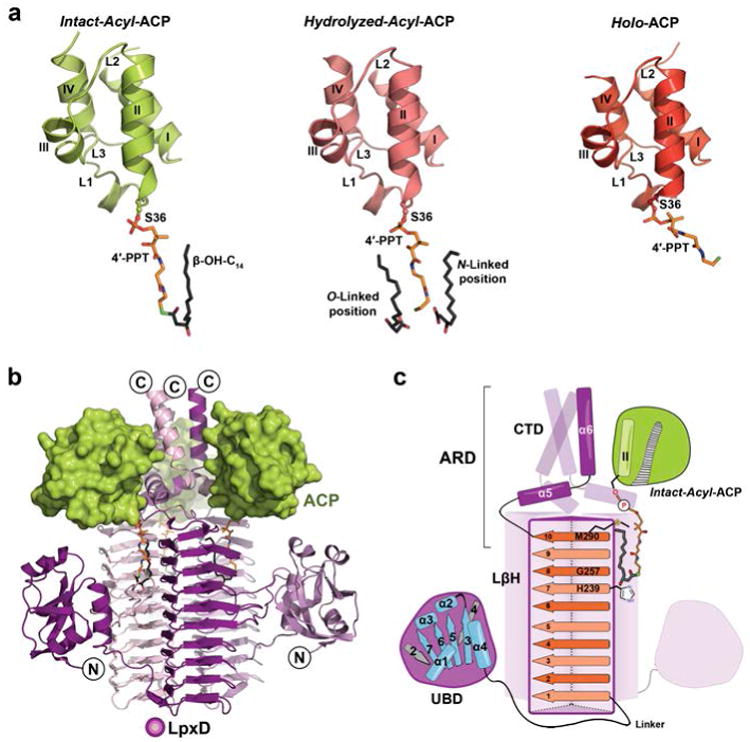
(a) The three forms of ACP: intact-acyl-ACP (green), hydrolyzed-acyl-ACP (salmon), and holo-ACP (red). ACP adopts a 4-helix bundle (I-IV) with several loop regions (L1-L3)28,29. The 4′-PPT arm and acyl-chains are shown as stick models and colored orange or dark gray, respectively, and by atom. (b) Overall architecture of the ACP-LpxD complex (intact-acyl-ACP shown). Three ACPs bind the carboxy-terminal end of the LpxD trimer (colored by chain purple/magenta/light pink). (c) Cartoon rendering showing the overall fold and interaction of acyl-ACP with LpxD. Highlighted dark purple is a single monomer of LpxD with subdomains indicated: uridine binding domain (UBD), left-handed β-helix domain (LβH), and C-terminal domain (CTD). ACP, interfacing with the ACP recognition domain (ARD), and its acyl-4′-PPT group are shown. The locations of the catalytic base (His239), oxyanion hole (Gly257), and molecular ‘hydrocarbon ruler’ (Met290) are indicated within the ten β-helical coils (orange strands) of a single LβH.
