Abstract
Background
The offspring of mothers with mood disorders may evidence increased behavioral problems as early as preschool; however, no study to date has examined psychophysiological characteristics during infancy, particularly among offspring of mothers diagnosed with bipolar disorder. Elucidating psychobiological mechanisms of risk early in development is critical to inform prevention and early intervention efforts.
Method
This study compared physiological and behavioral responsivity in 6-month-old infants (N=329) of mothers with lifetime histories of bipolar disorder (BD, n=44), major depressive disorder (MDD, n=244), or no history of Axis I disorders (CTL, n=41). Infant respiratory sinus arrhythmia (RSA) was measured in a laboratory stressor paradigm. Measures of infant affect and behavior during mother-infant interaction, current maternal depressive symptoms, and exposure to stressful life events were examined with respect to diagnostic group and RSA.
Results
Groups did not differ in baseline RSA or infant affect measures. However, during the stressor task, infants of mothers with BD evidenced increases in RSA, while infants of MDD and CTL mothers evidenced decreases in RSA. Though levels of postnatal stress and current levels of maternal depressive symptoms differed among groups, neither of these factors predicted infant psychophysiological responses.
Conclusions
At 6 months of age, infants of mothers with BD show differences in psychophysiological regulation. These differences cannot be accounted for by perinatal outcome, current maternal depressive symptoms or exposure to stressful life events, and thus may reflect endophenotypic markers of psychopathological risk.
Keywords: Bipolar, depression, RSA, heart rate, vagal, stress, infants, human
Despite increasing understanding of genetic mechanisms that mediate offspring risk for mood disorders (e.g., Craddock & Sklar, 2009), intrafamilial transmission remains poorly understood. Compared to offspring of healthy parents, offspring of patients with bipolar disorder evidence higher rates of bipolar spectrum disorders (Birmaher et al., 2009), manic and hypomanic symptoms (Findling et al., 2005), and internalizing disorders, including mood, anxiety and sleep disturbances (Duffy, Alda, Hajek, Sherry, & Grof, 2010). The deleterious impact of parental major depressive disorder on offspring outcomes has been widely studied, and data suggest deficits in emotion regulation and affective synchrony starting in infancy (Tronick & Reck, 2009) and increased prevalence of adverse psychiatric outcomes (Beardslee, Gladstone, & O’Connor, 2011; Goodman & Gotlib, 1999). However, data examining infants of patients with bipolar disorder appears lacking.
This study’s goal was to compare behavioral and physiological measures among infants of mothers with bipolar disorder, major depressive disorder and psychiatrically healthy controls, specifically focusing on respiratory sinus arrhythmia (Diwadkar et al.). RSA is a naturally-occurring variation in heart rate that occurs in synchrony with respiration, reflecting the balance of sympathetic and parasympathetic regulation of heart rate (Berntson, Cacioppo, & Quigley, 1993; Berntson et al., 1997). RSA is used as a laboratory index of physiological stress responsivity and regulation (Porges, 1995, 1997), and appears to be heritable (Snieder, Boomsma, Van Doornen, & De Geus, 1997; Wang, Thayer, Treiber, & Snieder, 2005), stable over time (El-Sheikh, 2005) and stable across tasks (e.g., Sloan et al., 1995) Levels of RSA at rest and the degree of RSA change in response to a stressor vary naturally in the population; individual differences in these measures have been associated with both major depressive disorder (e.g., Rottenberg, Clift, Bolden, & Salomon, 2007) and bipolar disorder (Cohen et al., 2003; Gruber, Dutra, Eidelman, Johnson, & Harvey, 2011; Gruber, Harvey, & Purcell, 2011) in adults, although findings are mixed, particularly in the depression literature (for review, see Rottenberg, 2007). There is some indication that RSA associations with depression are stronger in patients with a history of suicidal ideation (Chang et al., 2012a, 2012b), which may offer further support of RSA patterns as an index of dysregulation in patients with mood disorders.
Theoretical and empirical evidence suggests that social and behavioral deficits in mood disorders may be partially mediated by RSA via the vagus nerve (see Porges & Furman, 2011). Lower baseline values and failure to show RSA decreases in response to a stressor reflect less adaptive physiological indices (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996). Individual differences in RSA at baseline and in response to a stressor are evident early in development. Research on infants of depressed mothers, for example, shows that infants with lower baseline RSA evidence greater difficulties regulating their affective state (see Field & Diego, 2008 for review). RSA reactivity has also been identified as a potentially important biological marker in children with familial risk for mood disorders. At-risk children who showed a greater RSA decrease in response to a sad film clip showed better emotion regulation and less depressive symptoms on a clinician rated measured (Gentzler, Santucci, Kovacs, & Fox, 2009). Offspring of parents with bipolar disorder also report elevated levels of episodic and chronic stress, even after accounting for their own levels of affective symptoms (Ostiguy et al., 2009). Thus, exposure to stress could mediate associations between parental history of mood disorders and offspring outcomes.
The current study compares three groups of 6-month-old infants: those whose mothers have 1) a lifetime diagnosis of bipolar disorder (BD), 2) a lifetime diagnosis of Major Depressive Disorder (MDD) and 3) no history of an Axis I mental disorder (CTL). The primary aim was to examine group differences in infant baseline RSA and RSA reactivity over the course of a laboratory stressor. We hypothesized that infants of women with BD, compared to controls would demonstrate lower baseline RSA values and fail to show a decrease in RSA in response to a stressor. We compared levels of negative affect displayed during the stressor, and examined whether these may account for hypothesized physiological differences. We hypothesized that infants of mothers with BD would be exposed to greater levels of stress and maternal symptomatology, and we tested whether these environmental factors mediated the hypothesized RSA associations with maternal illness. Though mood dysregulation is also a feature of MDD, the mixed RSA literature and more heterogeneous nature of MDD hindered our ability to make specific, empirically-driven hypothesis with respect to infants of MDD mothers; thus, they were predicted to fall between the BD and CTL groups.
Methods
Participants
Women and their infants were recruited by the Emory Women’s Mental Health Program (WMHP) and the Emory University Psychology Department. Women with a lifetime history of a mood disorder were recruited by the WMHP from community referrals. Healthy volunteers were recruited by the Psychology Department via mass mailing. Inclusion criteria were: 1) maternal lifetime history of BD, MDD, or no Axis I disorder, and 2) consent by the mother for the maternal-infant dyad to complete the laboratory protocol. Subjects were excluded for: 1) maternal lifetime diagnosis of schizophrenia, 2) maternal diagnosis of substance abuse or dependence during pregnancy, and 3) major congenital malformations /disorders (e.g., cerebral palsy). The Emory University IRB approved this study, and participants provided written informed consent prior to participation.
Procedures
Participants arrived at 1:00 PM and were escorted to the Emory University Early Development Lab for the duration of the study. The protocol included: 1) Baseline, 2) Arm Restraint Stressor, 3) Maternal Interview. RSA data were collected during baseline and in the context of the arm restraint task, as described in detail below.
Prior to the collection of RSA data the mother held the infant while electrodes were placed on the center of the infant’s forehead and on each side of the chest. The infant was placed in a car seat in a quiet, low-lit room, while the mother sat behind an occlusion screen. Baseline heart rate data were then collected. During the Arm Restraint Stressor, the infant remained in the car seat. A research assistant gently held the infant’s arms down for up to two minutes while maintaining an emotionally neutral face toward the infant. If the infant cried continuously for 20 seconds, the arm restraint was stopped. The research assistant maintained the neutral expression for an additional minute after releasing the infant’s arms. The infant was videotaped during the arm restraint task, and his or her negative affect was coded offline by coders masked to maternal diagnostic status and trained by co-author Brennan. Approximately 10% of videotapes were recoded to demonstrate acceptable reliability (kappas > 0.70). Facial affect was coded continuously as positive, negative or neutral using Mangold INTERACT software. Negative affect during arm restraint was used as a potential mediating variable in the current study. Negative affect was defined as expressions of anger, disgust, contempt, sadness, pain, sympathy, or fear, and included observable behaviors such as mouth turned down, furrowed brow, wincing, scrunching up face, and crying.
The ECG signal collected at 250 Hz and was amplified and filtered by a bioamplifier (Coulbourn Instruments Model # V75-04, Allentown, PA). Power spectrum analysis software (Mindware Technologies, Lafayette Instruments Company, Gahanna, OH) was employed to determine high frequency heart rate variability using fast Fourier transformation to obtain the RSA (0.15–0.4 Hz). Correction of artifact, motion, and error in automated marking of R waves was performed manually. RSA was assessed during a 15-second baseline, and four additional 15-second time epochs in the arm restraint task: (A) the initial 15 seconds of restraint, (B) the final 15 seconds of restraint, (C) the first 15 seconds following release of the arms, and (D) the final 15 seconds of the 1-minute post-restraint period. RSA was computed as the average over the 15-second intervals, using peak to trough analysis in Mindware. While RSA is often calculated over a longer time epoch (30 seconds to several minutes), higher heart rates in infants relative to older children and adults enabled our ability to compute RSA in this shortened epoch and maintain a reliable estimate (Bar-Haim, Marshall, & Fox, 2000; Mezzacappa, Kindlon, Earls, & Saul, 1994). Statistical outliers (> 3 standard deviations above the mean) were Winsorized prior to data analysis.
Psychosocial Assessments
The following instruments were administered to the mother: 1) Structured Interview for DSM-IV (SCID; (First, Spitzer, Gibbon, & Williams, 2002) to identify current and lifetime maternal psychiatric diagnoses and thereby assign participants to a diagnostic group. The SCID was administered by Masters-level research assistants masked to maternal diagnostic history. A reliability analysis based on 10% of our sample rated by an independent judge yielded weighted kappas>0.75 for all anxiety and mood disorder diagnoses; 2) Beck Depression Inventory (BDI; (Beck, Steer, & Brown, 1996) to assess maternal depressive symptoms; 3) PERI Life Events Scale (PERI; (Dohrenwend, Krasnoff, Askenasy, & Dohrenwend, 1982) to rate the occurrence and severity of 35 stressful perinatal events on a 1 to 5 Likert scale (the summed total equaled the number of pregnancy and postpartum events multiplied by their average stress rating); 4) demographic questionnaire; and 5) obstetrical history questionnaire documenting medications during pregnancy and delivery complications (e.g., maternal hemorrhage, abnormal fetal position, abnormal fetal heart rate).
Table 1 presents descriptive statistics by group for the dependent and mediator variables examined in this study, as well as relevant demographics.
Table 1.
Mediator and sociodemographic variables by group
| Measure | BD Mean (SD) |
MDD Mean (SD) |
CTL Mean (SD) |
|---|---|---|---|
| Baseline RSA | 4.01 (2.20) | 4.04 (1.75) | 3.58 (1.76) |
| RSA A | 4.31 (1.83) | 3.70 (2.18) | 3.42 (1.71) |
| RSA B | 4.40 (2.95) | 3.22 (2.28) | 2.91 (2.43) |
| RSA C | 4.61 (2.92) | 3.55 (2.54) | 3.42 (2.62) |
| RSA D* | 4.44 (3.38) | 3.17 (2.25) | 2.81 (2.31) |
|
| |||
| Beck Depression Inventory* | 11.18 (10.55) | 10.16 (8.83) | 5.88 (4.44) |
| PERI Postpartum Stress* | 5.50 (6.02) | 2.95 (4.44) | 1.55 (2.65) |
| Infant Negative Affect During Stressor (%) | 56.65 (37.91) | 48.62 (39.35) | 56.81 (37.59) |
|
| |||
| Infant Age in Days* | 187.74 (15.08) | 180.08 (17.28) | 185.71 (14.39) |
| Infant Gender (% Male) | 59.1 | 53.3 | 58.5 |
| Gestational Age | 39.47 (1.02) | 39.06 (1.66) | 39.29 (1.47) |
| Infant Birth Weight (kg) | 3.31 (0.48) | 3.32 (0.55) | 3.33 (0.54) |
| Maternal Age* | 32.68 (4.05) | 34.13 (4.33) | 31.76 (4.82) |
| Marital Status (% Married/Partnered) | 81.8 | 93.8 | 92.7 |
p<0.05;
Note: Infant age is corrected for gestational age.
Data Analysis
Hypothesis testing used Hierarchical Linear Modeling (HLM6; Raudenbush & Bryk, 2002) procedures, which permit participant inclusion despite missing data points for dependent variables, and allow for an analysis of predictors of both intercept (i.e., baseline RSA) and slope (i.e., change in RSA in response to the arm restraint). Specifically, we analyzed a series of two level models, with repeated measurements of RSA (Level 1) nested within infant (Level 2). For model specification, the time parameter (dummy coded 0 to 4) was entered at Level 1, and maternal diagnostic group (coded 0 or 1) and statistical controls (as necessary, see below) were entered at Level 2. Levels of the outcome variable and the random effects modeled at Level 2 were allowed to vary across infants. All continuous variables at Level 2 were grand-mean centered, and all dichotomous variables at Level 2 were un-centered. Results were evaluated using final estimation of fixed effects with robust standard errors, which provide accurate significance tests under conditions of non-normality and in the presence of outliers and model misspecification (Raudenbush & Bryk, 2002).
It was hypothesized that maternal diagnostic group status would predict baseline RSA as well as growth trajectories of RSA throughout the course of the arm restraint stressor task. HLM models (equations) tested were the following:
- Level 1 Model
- Level 2 Model
- Mixed Model
Secondary analyses examining group differences in maternal postnatal stressful life events, maternal self-reported current depressive symptoms, and infant affect were performed using one-way ANOVA procedures. Tests of mediation were performed, in accordance with MacKinnon et al. (2002), using a test of joint significance.
Results
Description of Sample
The sample of 329 mother infant dyads was predominantly Caucasian (n=299, 91%), with 303 (92%) of mothers married or cohabitating. Mothers’ ages ranged from 20 to 44 years (M = 33.6, SD=4.4), with a median education level of college graduate. The infants included 180 (55%) males and 149 (45%) females with a mean age of six months, corrected for gestational age (SD= 16.6 days; range=4 months, 22 days to 7 months, 17 days). Women recruited from the clinic and the community did not differ with respect to race, education level, marital status, or employment status (ps>0.05). The sample included 44 mothers in the BD group (37 Bipolar I, 7 Bipolar II), 244 mothers in the MDD group, and 41 mothers in the CTL group. A majority of the sample (n=263, 80%) were euthymic based on SCID criteria (BD: n=45, 70.4%, MDD: n=191, =78.3%, CTL: n=41, 100%). Of the women diagnosed with unipolar or bipolar depression, 79.5% were taking psychotropic medications (predominately antidepressants and mood stabilizers). Among the mothers in the BD with active psychiatric illness, 8 were in a manic or hypomanic episode and 5 in a depressed episode.
Due to excessive infant movement, electrode placement difficulties, and equipment malfunction, RSA data were uncodeable for 80 (24.3%) of the infants, consistent with prior studies (Moore et al., 2009). These were excluded from RSA analyses. Infants in the CTL group were less likely to be excluded (7.3%) than infants in either the BD (31.8%) or MDD group (25.8%; χ2(N=329, df=2)=8.08, p=.02). Infants who were included in RSA analyses versus excluded did not differ in age, birth weight or length, Apgar scores, or delivery complications, and their mothers did not differ in age, current level of depressive symptoms, or stressors during pregnancy or postpartum (ps>0.10). Among the 249 infants included in the RSA analyses, there were no group differences in the number of RSA time points (F(2,248)=2.31, p=.10) or the length of the stressor task (F(2,248)=.93, p=.40), thus the missing-at-random assumption was satisfied. Due to videotape malfunction, affect data was missing for 77 (23%) infants. There were no diagnostic group differences in rates of missing videotaped observations (χ2 (N=329, df=2)=1.17, p=.56)
Preliminary Analyses
Preliminary analyses examined the impact of potential confounding variables, i.e., infant: gestational age at delivery, gender, age at study corrected for gestational age, birth weight, cesarean delivery, breast feeding at time of study, reflux, allergies, number of older siblings; mother: age, marital status, level of education, comorbid anxiety disorder, use of psychotropic medication during pregnancy, and postpartum medication exposure via lactation. Neither the intercept nor the slope of RSA was associated with these variables (ps>0.05). Infant asthma (n=2, both in MDD group) and current maternal mood episode polarity were also considered, but could not be tested statistically due to sample size. Given none of the covariates examined were associated with the dependent variables, they were not included in the analyses presented below.
Hypothesis Testing: Maternal Diagnosis and Infant RSA
“Empty” HLM models of RSA (i.e., those with no predictors) were examined using RSA levels obtained at baseline and throughout the arm restraint task. The time parameter was coded from 0 to 4, with 0 representing baseline. Therefore, equations predicting intercept were equivalent to tests of diagnostic group effects on baseline RSA. Results of the empty model were significant for a systematic negative linear change in infant RSA across time, t(245) = -2.90, p = .005 and the χ2 deviance test revealed no significant quadratic effects (χ2=1.68, p=ns). Significant between-subject variability in the time parameter (χ2 (204) = 299.98, p < .001) provided support for examining Level 2 predictors of change in RSA.
BD group status predicted the slope of RSA, with infants in the BD group having higher slopes than the other infants in the sample (Table 2). Follow up HLM models examining direct comparisons between groups demonstrated that RSA slopes were higher in the BD group than either of the other diagnostic groups. There were no maternal diagnostic group differences in baseline RSA (Table 3). For illustrative purposes, a graphical representation of the means over time by group is displayed in Figure 1.
Table 2.
Maternal diagnostic group and slope of infant RSA in response to a laboratory stressor
| Group Comparison (n) | Coefficient | SE | t | p |
|---|---|---|---|---|
| BD (30) vs. Other (219) | .53 | .19 | 2.82 | .006 |
| MDD (181) vs. Other (68) | -.11 | .06 | -1.81 | .07 |
| CTL (38) vs. Other (211) | -.02 | .15 | -0.13 | .90 |
| BD (30) vs. MDD (181) | .54 | .19 | 2.85 | .005 |
| BD (30) vs. CTL (38) | .50 | .23 | 2.17 | .03 |
| MDD (181) vs. CTL (38) | -.04 | .15 | -0.29 | .77 |
Table 3.
Maternal diagnostic group and infant baseline RSA
| Group Comparison | Coefficient | SE | t | p |
|---|---|---|---|---|
| BD (30) vs. Other (219) | -.35 | .54 | -0.64 | .52 |
| MDD (181) vs. Other (68) | .23 | .18 | 1.30 | .20 |
| CTL (38) vs. Other (211) | -.49 | .41 | -1.20 | .23 |
| BD (30) vs. MDD (181) | -.45 | .54 | -0.83 | .41 |
| BD (30) vs. CTL (38) | .08 | .64 | 0.13 | .90 |
| MDD (181) vs. CTL (38) | .53 | .41 | 1.29 | .20 |
Figure 1. Graphical representation of means over time for illustrative purposes.
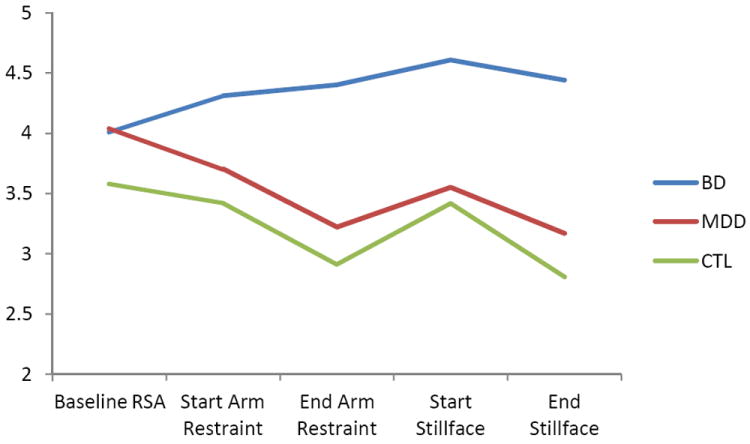
RSA reflects the 15 second epoch at the start of the arm restraint, RSA B reflects the last 15 second of the arm restraint, RSA C reflects the 15 second epoch at the start of the still face, and epoch D represents the last 15 seconds of the still face.
Hypothesis Testing: Maternal Depression and Stressful Life Events
ANOVA revealed maternal diagnostic group differences in the cumulative severity of stressful events during the postpartum period (F(2,328)=8.78, p<.001) and current BDI scores (F(2,312)=4.92, p=.008). Post hoc Duncan analyses revealed that BD mothers reported greater postpartum stress than MDD or CTL mothers, and that both MDD and BD mothers demonstrated more severe depressive symptoms than CTL mothers (see Table 1).
Because postpartum stress exposure and current depressive symptoms were associated with maternal diagnostic group, we tested whether they fulfilled the second criterion of a mediator (i.e., a significant relationship to the dependent variable of interest when accounting for the independent variable). HLM linear growth models revealed no significant effects of postpartum stress or maternal current depressive symptoms on the slope of infant RSA (ps<.15). Furthermore, infants of mothers who were euthymic (n=263) did not differ on RSA measures when compared to infants of mothers experiencing a current depressive (n=58) or manic episode (n=8; ps>.15). Thus, measures of maternal depressive symptoms, mood episode, and stressful life events failed to satisfy the criteria as mediators in the relationship between maternal diagnostic group status and infant changes in RSA.
Infant Behavioral Analysis
One-way ANOVA identified no differences between MDD, BD and CTL diagnostic groups in the percent of time that infants displayed negative affect during the arm restraint stressor task (F(2,251)=1.01, p=.37; see Table 1 for means and SDs). In addition, a supplemental HLM model revealed no association between the infants’ negative behavioral response to the arm restraint task and either baseline (t(177)=-0.15, p=.89) or change in RSA (t(177)=-1.62, p=.11).
Discussion
Our laboratory data supported the hypothesis that infants of BD mothers would differ from infants of CTL mothers in their physiological responses to stress. Specifically, infants of BD mothers showed an increase in RSA during a stressor task whereas infants of MDD or CTL mothers demonstrated a compensatory decrease in RSA. Group differences in infant behavior were not evident during the task. Although our measure of infant behavior may have lacked sensitivity, the absence of behavioral differences reduces the likelihood that RSA findings were due to movement, crying, or other behavioral factors. Unlike the other groups, infants of mothers with BD evidenced an increase in RSA in response to the arm restraint task, reflecting a failure of vagal withdrawal, which has been associated with poor behavioral and attentional regulation in infants (Calkins, 1997). These findings indicate that emotion regulation differences previously noted in the behavior of toddlers of mothers with BD (Gaensbauer, Harmon, Cytryn, & McKnew, 1984; Zahn-Waxler, Chapman, & Cummings, 1984), may be mediated by physiological dysregulation present from six months of age. Post-hoc analyses (data not shown) suggested that prenatal exposure effects of maternal symptoms were not the driving force behind our significant findings. These maternal symptom data were obtained retrospectively through the SCID; prospective studies would be needed to confirm these results. Longitudinal data are needed to understand the developmental implications of our findings, and studies that combine measures of infant temperament along with behavioral and physiological data would make an important contribution.
In contrast to RSA reactivity, we did not find between-group differences in baseline RSA. Our data are thus consistent findings of RSA in adults with BD, which highlight abnormal RSA reactivity in response to emotional stimuli rather than baseline differences (Gruber, Harvey, et al., 2011). Thus, RSA change, which reflects a person’s flexible adaption to the environment, may be more relevant to mood dysregulation, a hallmark of bipolar disorder. Twin studies have revealed greater concordances for RSA during a stressful task than for RSA at rest (Boomsma, Baal, & Orlebeke, 1990), offering further support of RSA reactivity as an endophenotypic marker. Some methodological issues may have also hindered our ability to detect baseline differences, i.e., “baseline” RSA was recorded immediately after the placement of electrodes and with the mother standing out of the infant’s line of sight. While not a significant and reliable stressor like the arm restraint, this may have been mildly stress-inducing for a subset infants, thus hindering our ability to detect group differences in baseline data.
This sample of mothers with BD reported higher levels of stressful life events during the postpartum period than MDD mothers or control mothers, consistent with recently published findings on older offspring (Ostiguy et al., 2009). Current stress levels were unrelated to RSA levels, suggesting that physiological regulation differences in infants of BD mothers were not accounted for by their exposure to a stressful environment. Measures of daily stress or hassles experienced by the mother or poor maternal coping skills in response to stress could provide other avenues of physiological risk; this possibility warrants further exploration. Similarly, current depressive symptom levels did not mediate associations between maternal diagnostic status and infant RSA. Further, infant RSA measures did not differ between mothers who were euthymic at the time of testing compared to those who were not; thus current maternal mood state, assessed either categorically or continuously, did not account for the observed differences in infant RSA. Future studies should consider including measures of maternal RSA to better understand the contribution of the mother’s current state to the physiological and behavioral responsivity of the infant.
One limitation of this study is the lack of a self-reported rating scale of manic symptom, which would have allowed us to explore more specific associations between maternal mood symptoms and infant RSA in the BD group. Additional symptom measures, including those related to anxiety and mania, have been added to future studies. The lack of information with respect to paternal psychiatric status and mental health history is also a limitation, though feasibility concerns hinder our ability to also interview fathers, especially given inherent demands on the family of a young infant. Additional studies are collecting data from fathers for genotyping, in order to better understand genetic factors that contribute to intergenerational transmission of psychiatric illness. The homogenous sample may be seen as another limitation, in that it can hinder generalizability of findings. However, the fact that sample characteristics did not differ among groups facilitates interpretation of group differences. Data loss is common in studies of infants using psychophysiological measures and while issues of sampling bias and should always be considered, there was no evidence to suggest that missing data influenced the current findings.
Given that stress and maternal symptomatology did not mediate RSA-associations, disruptions in infant RSA reactivity may reflect a developmental endophenotype associated with offspring risk for mood disorder. Genetically-sensitive studies are needed to further address these questions, and longitudinal data will be crucial in determining clinical and behavioral implications of the observed physiological differences. Findings from the current study make an important contribution to the understanding of risk transmission in mood disorders. To our knowledge, this is the first study to examine RSA in offspring of mothers with BD and the only study we have identified that measures infant physiology and behavior in that population. Abnormal RSA reactivity in the absence of observed behavioral differences highlights the value of physiological measures as potential indicators of risk. The fact that these physiological differences are observed at six months of age, years prior to the emergence of any clinical diagnoses, further underscores the unique contribution of our data. While preliminary, these findings may ultimately have implications for early intervention. Specific strategies to scaffold appropriate affect regulation and minimizes environmental stressors that may contribute to infant dysregulation could change the developmental trajectory of at-risk offspring. Studying physiological regulation in infants provides a critical window into mechanisms of risk transmission.
Key points.
Offspring of mothers with mood disorders evidence increased risk for negative clinical outcomes; elucidating psychobiological mechanisms of risk early in development is critical.
Respiratory sinus arrhythmia (RSA) was measured in a laboratory stressor paradigm in infants of mothers with and without mood disorders to assess physiological reactivity to stress in a high-risk sample.
Infants of mothers with bipolar disorder show a maladaptive pattern of psychophysiological regulation in response to stress, which was not accounted for by perinatal outcome, maternal depressive symptoms, or stressful life events.
These findings suggest that RSA may reflect an endophenotypic marker of psychopathological risk evident very early in development, prior to evidence of any clinical signs or symptoms and support the need for longitudinal data to better understand clinical implications.
Acknowledgments
This study was supported by Brain and Behavior Research Foundation awards to Drs. Brennan and Johnson, the Emory University Silvio O. Conte Center for the Neurobiology of Mental Disease (MH58922), the Specialized Center of Research (SCOR) on Sex and Gender Effects (MH68036), and the National Institute of Mental Health (MH71531, MH88609).
Dr. Johnson has received research support from NARSAD. Dr. Brennan has received research support from NARSAD and the National Institutes of Health (NIH). Dr. Stowe has received research support from, and consulted to GlaxoSmithKline, Pfizer, and Wyeth Corporations, and received speakers’ honoraria from the same companies plus from Eli Lilly and Forest Corporations. Dr. Leibenluft receives research support from the NIMH intramural program. Dr. Newport has received research support from NARSAD and the National Institutes of Health (NIH), as well as Eli Lilly, GlaxoSmithKline (GSK), Janssen, and Wyeth Corporations, and speaker’s honoraria from Astra-Zeneca Pharmaceuticals (AZP), Eli Lilly, GSK, and Pfizer Corporations. No coauthor or any family member holds equity positions in pharmaceutical or biomedical corporations.
Footnotes
Conflict of interest statement: The authors have declared that they have no competing or potential conflicts of interest.
References
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37(1):44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Gladstone TRG, O’Connor EE. Transmission and Prevention of Mood Disorders Among Children of Affectively Ill Parents: A Review. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(11):1098–1109. doi: 10.1016/j.jaac.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Thomas Bigger J, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Van Der Molen MW, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Brent D, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: The Pittsburgh bipolar offspring study. Archives of General Psychiatry. 2009;66(3):287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, Baal GCMv, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. 1990 doi: 10.1017/s0001566000005419. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31(2):125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125∷aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Chang H-A, Chang C-C, Chen C-L, Kuo TBJ, Lu R-B, Huang S-Y. Heart rate variability in patients with fully remitted major depressive disorder. Acta Neuropsychiatrica. 2012a doi: 10.1111/j.1601-5215.2012.00658.x. no-no. [DOI] [PubMed] [Google Scholar]
- Chang H-A, Chang C-C, Chen C-L, Kuo TBJ, Lu R-B, Huang S-Y. Major depression is associated with cardiac autonomic dysregulation. Acta Neuropsychiatrica. 2012b doi: 10.1111/j.1601-5215.2011.00647.x. no-no. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M, Mittelman I, Osher Y, Bersudsky Y. Impaired heart rate variability in euthymic bipolar patients. Bipolar Disorders. 2003;5(2):138–143. doi: 10.1034/j.1399-5618.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends in Genetics. 2009;25(2):99–105. doi: 10.1016/j.tig.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Keshavan MS, et al. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: Comparing vulnerability markers. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(5):1349–1354. doi: 10.1016/j.pnpbp.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. The psychiatric epidemiology research interview life events scale. In: Goldberger L, Breznitz S, editors. Handbook of stress: Theoretical and clinical aspects. New York: Free Press; 1982. [Google Scholar]
- Duffy A, Alda M, Hajek T, Sherry SB, Grof P. Early stages in the development of bipolar disorder. Journal of Affective Disorders. 2010;121(1–2):127–135. doi: 10.1016/j.jad.2009.05.022. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology. 2005;46(1):66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M. Vagal activity, early growth and emotional development. Infant Behavior and Development. 2008;31(3):361–373. doi: 10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, McNamara NK, Stansbrey RJ, Demeter CA, Bedoya D, Calabrese JR. Early symptoms of mania and the role of parental risk. Bipolar Disorders. 2005;7(6):623–634. doi: 10.1111/j.1399-5618.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department New York State Psychiatric Institute; 2002. [Google Scholar]
- Gaensbauer TJ, Harmon RJ, Cytryn L, McKnew DH. Social and affective development in infants with a manic-depressive parent. Am J Psychiatry. 1984;141(2):223–229. doi: 10.1176/ajp.141.2.223. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gruber J, Dutra S, Eidelman P, Johnson SL, Harvey AG. Emotional and physiological responses to normative and idiographic positive stimuli in bipolar disorder. Journal of Affective Disorders. 2011;133(3):437–442. doi: 10.1016/j.jad.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Purcell A. What goes up can come down? A preliminary investigation of emotion reactivity and emotion recovery in bipolar disorder. Journal of Affective Disorders. 2011;133(3):457–466. doi: 10.1016/j.jad.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Kindlon D, Earls F, Saul JP. The utility of spectral analytic techniques in the study of the autonomic regulation of beat-to-beat heart rate variability. International Journal of Methods in Psychiatric Research 1994 [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, Cox MJ. Mother–Infant Vagal Regulation in the Face-To-Face Still-Face Paradigm Is Moderated by Maternal Sensitivity. Child Development. 2009;80(1):209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Ostiguy CS, Ellenbogen MA, Linnen AM, Walker EF, Hammen C, Hodgins S. Chronic stress and stressful life events in the offspring of parents with bipolar disorder. Journal of Affective Disorders. 2009;114(1-3):74–84. doi: 10.1016/j.jad.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: An Evolutionary By-Product of the Neural Regulation of the Autonomic Nervous Systema. Annals of the New York Academy of Sciences. 1997;807(1):62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29(8):697–712. doi: 10.1002/(sici)1098-2302(199612)29:8<697∷aid-dev5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behaviour: a polyvagal perspective. Infant and Child Development. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Application and data analysis methods. 2. Thousand Oaks: Sage; 2002. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44(3):450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Fishkin PE, Gorman JM, Myers MM. Consistency of heart rate and sympathovagal reactivity across different autonomic contexts. Psychophysiology. 1995;32(5):452–459. doi: 10.1111/j.1469-8986.1995.tb02096.x. [DOI] [PubMed] [Google Scholar]
- Snieder H, Boomsma DI, Van Doornen LJP, De Geus EJC. Heritability of respiratory sinus arrhythmia: Dependency on task and respiration rate. Psychophysiology. 1997;34(3):317–328. doi: 10.1111/j.1469-8986.1997.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Tronick E, Reck C. Infants of Depressed Mothers. Harvard Review of Psychiatry. 2009;17(2):147–156. doi: 10.1080/10673220902899714. [DOI] [PubMed] [Google Scholar]
- Wang X, Thayer JF, Treiber F, Snieder H. Ethnic Differences and Heritability of Heart Rate Variability in African- and European American Youth. The American Journal of Cardiology. 2005;96(8):1166–1172. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Chapman M, Cummings EM. Cognitive and social development in infants and toddlers with a bipolar parent. Child Psychiatry and Human Development. 1984;15(2):75–85. doi: 10.1007/bf00706165. [DOI] [PubMed] [Google Scholar]


