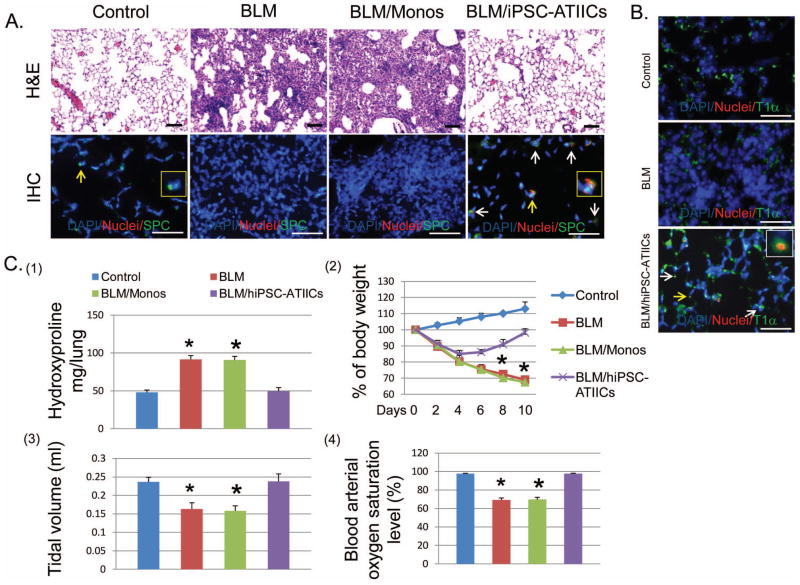
Figure 5. Functional repair of BLM-injured lungs by transplanted hiPSC-ATIICs in mice.
(A) Hematoxylin–eosin (H&E) staining of representative BLM-injured lung sections (upper panel, scale bar = 200 μm) showed that BLM-challenge caused severe alveolar architecture damage in 73.1 ± 5.3% of lung area with extensive inflammatory infiltrates and edema (the second image) compared with saline control (the left image). Functional transplantation of hiPSC-ATIICs was noted by significantly reduced alveolar structure damage in only 6.2 ± 2.3% of lung tissue (the right image). In contrast, the severity of alveolar structure damage in the BLM-challenged lung transplanted with hmonos (71.8 ± 5.9%) was similar to BLM-challenged lungs without hiPSC-ATIIC transplantation (the third image). Immunofluorescent staining of representative lung sections from BLM-challenged mice with and without transplanted hiPSC-ATIICs or hmonos was performed by using a mouse anti-human nuclei monoclonal antibody and rabbit anti-human proSPC antibody with DAPI counterstaining (lower panel, scale bar = 100 μm). The human nuclei monoclonal antibody recognizes cells of human origin in mouse lungs (red); the rabbit anti-human proSPC stains both mouse and human ATIICs that express SPC (green). The normal distribution of mouse ATIICs was observed in control lungs (the first image). A magnified view of a SPC positive cell indicated by the yellow arrow is inserted. In compared to control lungs, no ATIICs existed in BLM-injured lung area of mice received either saline or hmonos (the second and the third image). Human nuclei positive cells (red) were identified only in the BLM-challenged lungs transplanted with hiPSC-ATIICs. Some of these human nuclei positive cells (red) were co-stained with SPC (green), representing remained hiPSC-ATIICs (the right image, indicated by arrows) in the injured lungs. The magnified view of a human nuclei positive and SPC positive cell indicated by the yellow arrow is inserted. Those human nuclei positive cells that were SPC negative in the BLM-injured lungs suggested that some of transplanted hiPSC-ATIICs had differentiated into ATICs in the lungs. (B) Immunofluorescent staining of representative lung sections from BLM-challenged mice with and without transplanted hiPSC-ATIICs was performed by using a mouse anti-human nuclei monoclonal antibody and 1:50 diluted rabbit anti-human T1α antibody (Abgent, San Diego, CA), with DAPI counterstaining, for identifying human derived ATICs. The rabbit anti-human T1α antibody stains both mouse and human ATICs that express T1α (green). The T1α positive cells were observed in control lungs (control panel, scale bar = 100 μm) and BLM-challenged lings (BLM panel). Human nuclei positive cells (red) were identified only in the BLM-challenged lungs transplanted with hiPSC-ATIICs (BLM/hiPSC-ATIICs panel). Some of these human nuclei positive cells were co-stained with T1α (indicated by the arrows), indicating the cells with ATIC phenotype derived from the transplanted hiPSC-ATIICs in the injured lungs. A magnified view of a human derived cell with ATIC phenotype indicated by yellow arrow was inserted in the image. (C) (1). Hydroxyproline levels in BLM-challenged lungs (91.56 ± 4.93 μg/lung) were significantly increased compared with that in saline control lungs (47.90 ± 3.30 μg/lung) at end of time point. Transplantation of hiPSC-ATIICs significantly reduced the content of hydroxyproline (49.71 ± 4.46μg/lung) in BLM-challenged lungs to the control levels. Consistent with histological analysis, transplanted hmonos did not reduce collagen deposition (90.7 ± 4.87 μg/lung) in the BLM-challenged lungs (n=8). (2). During the time course of BLM-challenge, body weight of BLM-challenged mice received either saline or hmonos significantly decreased over time and was down to ~70% of their initial body weight at end of time point. Although BLM-challenged mice that received hiPSC-ATIICs experienced an initial body weight loss, they started their body weight recovery on day 6 after lung injury and had recovered ~98% of their initial body weight on day 10 (n=8). (3). The lung tidal volumes were measured in BLM-challenged mice with and without transplantation of hiPSC-ATIICs or hmonos at end of time point. BLM-challenge caused a significant decrease in tidal volume (0.163 ± 0.017 ml) compared to saline control (0.236 ± 0.013 ml). In comparison, transplanted hiPSC-ATIICs (0.238 ± 0.020 ml), but not transplanted hmonos (0.158± 0.013 ml), restored tidal volume in BLM-challenged mice to the control levels. (n=8) (4). The blood arterial oxygen saturation levels were recorded in BLM-challenged mice with and without transplantation of hiPSC-ATIICs or hmonos at end of time point. The blood arterial oxygen saturation levels significantly decreased in BLM-challenged mice (69.25 ± 2.11%) and BLM-challenged mice that received hmonos (69.66 ± 2.44%) compared to that in saline control mice (97.73 ± 0.33%). In contrast, BLM-challenged mice transplanted with hiPSC-ATIICs had completely recovered normal arterial oxygen saturation levels (97.66 ± 0.49%) (n=8). * P<0.001 versus BLM/hiPSC-ATIICs and control group.