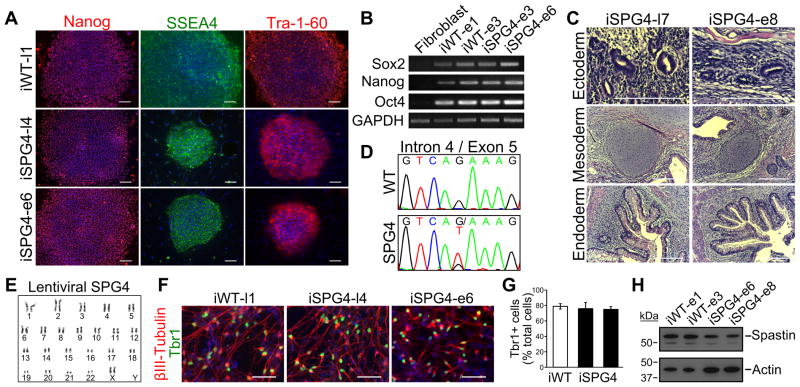
Figure 1.
Characterization and differentiation of SPG4 iPSCs. (A) Control (iWT-l1, lentiviral) and SPG4 iPSC lines expressed the hESC markers Tra-1-60, Nanog and SSEA-4. Scale bars: 100 μm. (B) Analysis of pluripotency genes in generated iPSC lines (iWT-e1, iWT-e3, iSPG4-e3, iSPG4-e6, episomal) by RT-PCR. (C) Hematoxylin and eosin staining of teratoma sections that were derived from iPSCs. Tissues from each germ layer were formed. Scale bars: 200μm. (D) Sequencing results confirmed the presence of the intron 4 splice acceptor mutation (c.683-1G>T), which was not found in control cells. (E) The lentiviral SPG4 lines maintained a normal 46,XX female karyotype after 10 passages as shown by G-banded analysis. (F) The iPSCs were able to efficiently generate telencephalic glutamatergic neurons, as shown by staining for Tbr1. Scale bars: 50μm. (G) Percentages of cells immunostained positive for Tbr1 in control (iWT-l1) and SPG4 lines (iSPG4-l4 and iSPG4-l7). Data presented as mean ±SD. (H) Western blot analysis revealed a significant decrease in spastin protein in post-mitotic neurons derived from SPG4 iPSCs compared to control neurons. Nuclei are labeled with Hoechst (blue) in A and F.