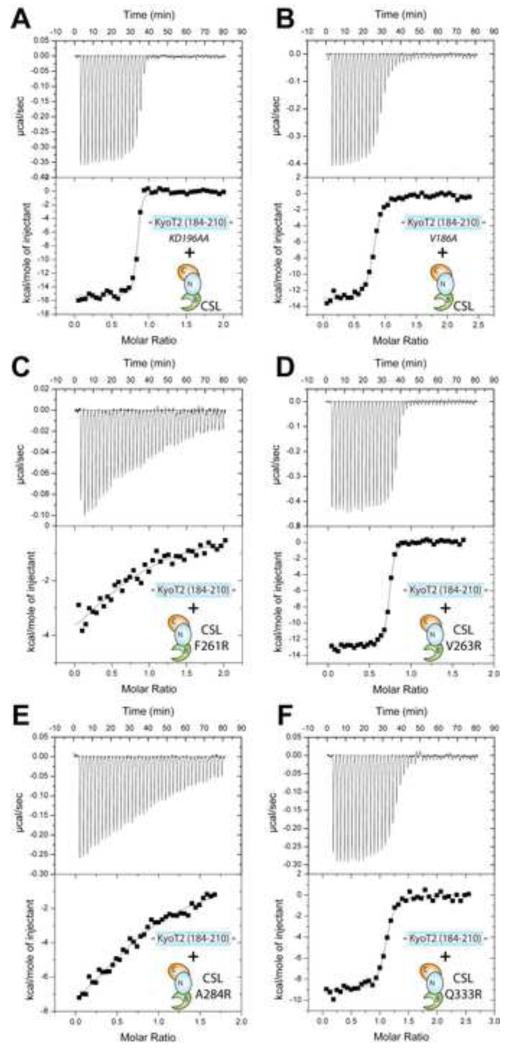
Figure 5. Thermodynamic binding analysis of KyoT2 and CSL mutants.
This figure shows representative thermograms (raw heat signal and nonlinear least squares fit to the integrated data) for KyoT2 mutants KD196AA (A) and V186A (B) binding to wild-type core CSL; and CSL mutants F261R (C), V263E (D), A284R (E), and Q333R (F) binding to wild-type KyoT2 (184-210). Forty titrations were performed per experiment, consisting of 7 μl injections that were spaced 120s apart.