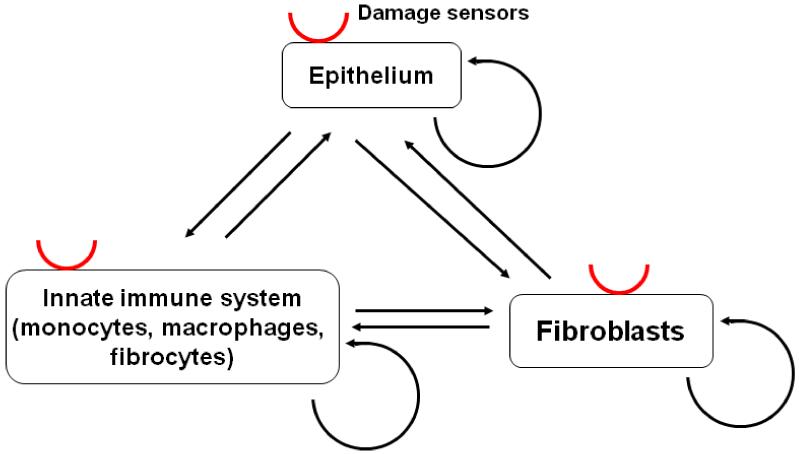
Figure 1.
Diagram of the known and potential interactions among the key cell types that participate in fibrosis. Red semicircles indicate damage sensors. Arrows indicate known or potential signal interactions, which could be either stimulatory or repressive. Fibroblasts have the potential to proliferate, and these interactions evolved to have the capacity to do two quite different things. First, negative feedback loops must exist to maintain homeostasis and prevent fibroblasts from proliferating, or at least limit fibroblast proliferation to just replacing dead fibroblasts, in a healthy adult tissue such as the lung. Second, activating pathways evolved to allow damage or wounding (sensed by some combination of epithelial cells, the innate immune system, and fibroblasts) to trigger fibrocyte differentiation, fibroblast activation, and fibroblast proliferation, all of which participate in wound contraction and the formation of scar tissue. The system has thus had to evolve a tricky balancing actremain quiescent in the absence of wounding, but respond quickly and vigorously when there is damage or wounding, and then stop the response when the damage has been repaired. Fibrosis appears to be a situation where this balancing act gets stuck in the repair mode. A probable scenario for fibrosis involves some sort of initial damage, a response from the innate immune system and resident fibroblasts, and then a positive feedback loop forming where, for instance, innate immune system cells activate fibroblasts, causing some sort of stress which is sensed by epithelial cells as damage, and these cells then activating the innate immune system. There are thus many possible targets to slow or stop this vicious cycle, and thus slow or stop fibrosis.