Abstract
Regulatory T cells (Tregs) are instrumental in the induction and maintenance of tolerance, including in transplantation. Tregs induce allotolerance by interacting with antigen-presenting cells (APC) and T cells, interactions that require their proper homing to the lymphoid tissues. Using a well characterized model of corneal allotransplantation, we demonstrate here that Tregs in the draining LN of allograft acceptors, but not rejectors, colocalize with APC in the paracortical areas and express high levels of C-C motif chemokine receptor 7 (CCR7). In addition, we show that Treg expression of CCR7 is important not only for Treg homing to the draining LN, but also for optimal Treg suppressive function. Finally, we show that Tregs augmented for CCR7 expression by their ex vivo stimulation with the CCR7-ligand CCL21 show enhanced homing to the draining LN of allograft recipients and promote transplant survival. Together, these findings suggest that CCR7 expression is critical for Treg function and migration, and that conditioning of Treg for maximal CCR7 expression may be a viable strategy for promoting allograft survival.
Keywords: Regulatory T cells, CCR7, Tolerance, Transplantation, Cornea
INTRODUCTION
Regulatory T cells (Tregs) are master regulators of the immune system that have been shown to impact nearly every aspect of T cell function by interacting with both T cells and antigen-presenting cells (APC) (1–4). CD4+CD25+Foxp3+ Tregs (which constitute 5–10% of CD4+ T cells) have shown considerable promise in strategies for the induction of allo-specific tolerance in a number of settings including skin, cardiac, kidney and islet transplantation, with most experimental strategies focused on Treg expansion and perioperative transfer to grafted hosts (5–7).
The current paradigm of Treg function proposes that these cells function in two principal locations – secondary lymphoid tissues, where they suppress induction of immunity by regulating T cell priming and expansion, and inflamed peripheral tissues where they suppress effector immune cell functions (8–10). Appropriate Treg homing in secondary lymphoid tissues is required for the functional clustering of Tregs with APC and T cells, which is needed for the induction and maintenance of immunological tolerance (11, 12). This Treg localization does not occur randomly, but is a highly coordinated process involving chemokines and adhesion molecules. A majority of Tregs found in the secondary lymphoid compartment preferentially express L-selectin (CD62L) and CC-chemokine receptor-7 (CCR7) that regulate Treg extravasation across the high endothelial venules and homing to the lymphoid tissues (9, 13–15).
Using a well-characterized mouse model of corneal transplantation (16, 17), we recently provided new insights on Treg localization and function in transplantation immunity (18). We reported that Tregs are crucial in promoting corneal transplant (the most common form of tissue allografting) survival primarily by suppressing the induction of alloimmunity in the draining lymph nodes (LN). In addition, we demonstrated that allograft outcome was closely correlated to Treg suppressive function rather than Treg frequencies; indeed, while Treg frequencies in the draining LN were found to be similar in both corneal allograft-acceptors and rejectors, only Tregs in the LN of acceptors were capable of preventing allograft rejection (18). Importantly, however, the mechanisms that determine why Tregs in the LN of allograft acceptors are more functionally effective than those in allograft rejectors are not well understood.
In the present study, we investigated the localization of Tregs in the draining LN of allograft recipients, and evaluated the expression patterns of different homing receptors by these Tregs. Our data demonstrate that Treg expression of CCR7 is most critical in regulating both their homing and suppressive function. Moreover, we provide novel evidence that ex vivo conditioning of Tregs with CCL21 leads to significant upregulation of Treg expression of LN homing receptors, including CCR7, and these CCR7-amplified Tregs home more efficiently to the reactive LN of corneal allograft recipients to promote graft survival.
MATERIALS AND METHODS
Mice
Eight-week-old male BALB/c and C57BL/6 (Taconic Farms, Germantown, NY) mice were used in all experiments. CCR7−/− C57BL/6 mice were provided by Andrew Luster (Massachusetts General Hospital, MA). Mice were housed in a specific pathogen-free environment at the Schepens Eye Research Institute animal facility. All procedures were approved by the Institutional Animal Care and Use Committee, and all animals were treated according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Corneal transplantation
Standard protocol for murine orthotopic corneal transplantation was used, as described previously (16–18). Briefly, donor center corneas (2 mm diameter) were excised from C57BL/6 mice and sutured onto recipient graft beds prepared by excising a 1.5-mm site in the central cornea of BALB/c mice. Simultaneously, some BALB/c mice received syngeneic (BALB/c) grafts to control for the non-allospecific effects of surgery. The corneal sutures were removed 7 days after surgery. All grafts were evaluated using slit-lamp biomicroscopy at weekly intervals. Grafts were defined as rejected when they became opaque and the iris details could not be recognized clearly using a standardized opacity grading (range, 0–5) scheme (16–18). In this model, ~50% of allografts are rejected within 3 week of transplantation while the remaining half enjoys indefinite survival. Compared with other transplant systems (e.g., skin, heart, kidney, etc.) where a 100% graft rejection rate is observed in unmanipulated recipients, this model provides an exceptional opportunity to study why some allografts are spontaneously accepted and to evaluate the role of Tregs in this high frequency of transplant survival.
Treg isolation and culture
CD4+CD25+ Tregs from the lymph nodes were isolated by magnetic separation using regulatory T cell isolation kit (Miltenyi Biotec). Purity of sorted cells was > 97% as confirmed by Foxp3 staining using flow cytometry. Sorted Tregs were cultured in complete RPMI1640 media under standard culture conditions. To stimulate Tregs with chemokine ligands, recombinant murine CCL19 or CCL21 (Peprotech Inc) were added at a concentration of 1.0μg/ml (19) to either media alone or media with conditioning cocktail (CD3 Ab, CD28 Ab and IL-2) for Treg expansion (20).
Immunohistochemistry
Draining submandibular LN of different transplant recipients were harvested at 3 week post-transplantation and acetone-fixed cryosections were prepared for immunostaining. The sections were first blocked with 2% BSA and anti-FcR Ab (eBioscience), and then immunostained with primary anti-mouse Foxp3 and anti-mouse CD11c or isotype-matched control Abs (Santa Cruz Biotechnology) overnight at 4°C and then with Alexa Fluor 350- and rohdamine-conjugated secondary Abs (Invitrogen) for 1h at room temperature. The stained cross-sections were mounted using Vector Shield mounting medium (Vector Laboratories) and examined under the epifluorescence microscope (Nikon).
Flow cytometry
Draining LN were harvested from different transplant recipients, and single-cell suspensions were prepared (18). The isolated cells were stained with the following Abs: Anti-CD4 FITC, anti-FoxP3 PECy5, and anti-CCR7, -CD62L, or -CD103 PE (eBioscience). CD4+Foxp3+Tregs were gated first, and then expression of different receptors was analyzed using an EPICS XL flow cytometer (Beckman Coulter).
In vitro Treg suppression assay
Naïve CD4+CD25+ Tregs from the LN of wild type and CCR7 KO mice, and CD4+CD25-Teff from the LN of wild type were isolated using magnetic separation kit (Miltenyi Biotec). As described previously (18), CD4+CD25− Teff cells (1×105) were cocultured with CD4+CD25+ Tregs (5×104), T cell-depleted syngeneic splenocytes (1×105) and 1 μg/ml anti-CD3 antibody for 3 days. Proliferation of CD3 stimulated Teff cells without the addition of Tregs was considered as control proliferation with 0% suppression. Proliferation was measured using the BrdU incorporation assay (Millipore) and percent suppression was calculated using the following formula: (18). In addition, culture supernatants were collected to evaluate the expression of immunoregulatory cytokines IL-10 and TGF-b using commercially available ELISA kits (R & D Systems).
Adoptive transfer of Tregs to allograft recipients
Tregs were labeled with PKH-26 vital dye (Sigma Aldrich) as per the manufacturer’s instructions. PKH-26-labeled Tregs (2×105 cells per mouse) were transferred intravenously to the allograft recipients at 24h post-surgery. Allograft survival rate in each group (n = 8 per group) was monitored up to 8 weeks post-transplantation.
Statistical analysis
Student’s t test was used for comparison of mean between the groups. Kaplan-Meier analysis was adopted to construct survival curves, and the log-rank test was used to compare the rates of corneal graft survival. Data are presented as mean ± SEM and considered significant at P<0.05.
RESULTS
Tregs of allograft acceptors colocalize with APC in the paracortical areas of draining LN and express higher levels of CCR7
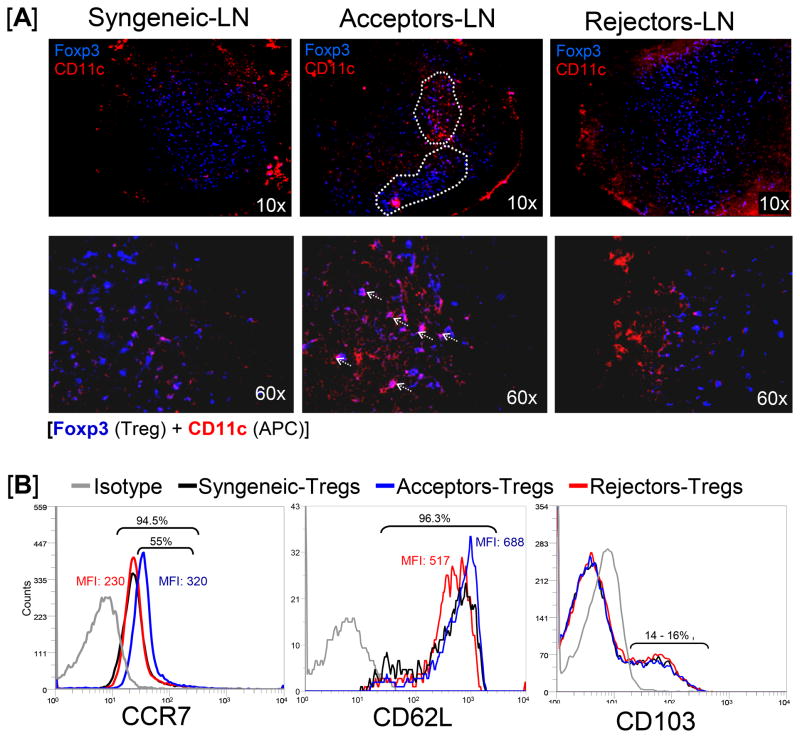
In order to investigate why Tregs are functionally more effective in allograft acceptors than rejectors, we first investigated the localization and cellular interactions of Tregs in the regional LN of grafted hosts. Draining LN from syngeneically grafted recipients (Balb/c→Balb/c) and fully disparate allograft (C57Bl/6→Balb/c) acceptors and rejectors were harvested and immunostained for Foxp3+ Tregs and CD11c+ antigen presenting cells (APC) (Fig 1A). In allograft acceptors, Foxp3+ Tregs were localized in focal clusters in the paracortical region, primarily in close proximity to CD11c+ APCs. In contrast, in the LN of allograft rejectors and syngeneically grafted recipients, Foxp3+ Tregs were found evenly distributed throughout the paracortex and were rarely in contact or close proximity with CD11c+ APC.
Figure 1. Localization and homing receptor expression by Tregs in the draining LN of syngeneically grafted recipients (Balb/c→Balb/c) and fully disparate allograft (C57Bl/6→Balb/c) acceptors and rejectors.
The draining LN were harvested 3 weeks after corneal transplantation. In this model of corneal transplantation, ~50% of allografts are rejected within 3 weeks of transplantation while the remaining half enjoys indefinite survival. (A) Confocal micrographs of LN show that only the acceptor-Tregs (Foxp3, blue) are focally distributed in the paracortical area of the LN (circled with white dotted line [10x]) and colocalize with APC (CD11c, red) (marked with white arrows [60x]). (B) Levels of CCR7, CD62L and CD103 expressions by CD4+CD25+Foxp3+ Tregs were analyzed by measuring mean fluorescent intensity (MFI) using flow cytometry, showing that acceptor-Tregs express very high levels of CCR7 compared to those expressed by rejector- and syngeneic-Tregs. Each transplant group consists of 6 mice, and data from a representative experiment of two performed is shown.
To further understand why Tregs show different patterns of distribution and cellular interaction, we hypothesized that Tregs of allograft acceptors express high levels of homing receptors that enhance their homing and migration to the site of incoming APC in the reactive LN. We thus investigated Treg expression of CCR7, CD62L and CD103 homing receptors (Fig 1B). Single cell suspensions prepared from the reactive draining LN of different transplant recipients were analyzed using flow cytometry to determine the frequencies of CCR7+, CD62L+, and CD103+ Tregs, which were calculated by gating on CD4+FoxP3+Tregs. In addition, analysis of mean fluorescence intensity (MFI) provided levels of receptor expression by these Tregs. There was no difference in the frequencies of any of the homing receptor expressing Tregs in any of the transplant recipient groups – approximately all the LN Tregs (>95%) expressed CCR7 and CD62L, and only 14 – 16 % of Tregs expressed CD103. Interestingly, however, the Treg expression levels of CCR7 were substantially different among groups. More than 55% of Tregs from the LN of allograft acceptors expressed higher levels of CCR7 (MFI =320) compared to the Tregs of allograft rejectors and syngeneically grafted recipients, which expressed lower levels of CCR7 (MFI = 230). In contrast to CCR7 expression, no difference in CD62L expression level was observed between Tregs from allograft acceptors and syngeneically grafted recipients and Tregs from allograft rejectors expressed relatively low levels of CD62L. Thus, the differential expression of these Treg homing receptors suggested to us that CCR7 could be the critical homing receptor regulating Treg migration towards APCs in the LN.
CCR7 expression affects Treg phenotype and function
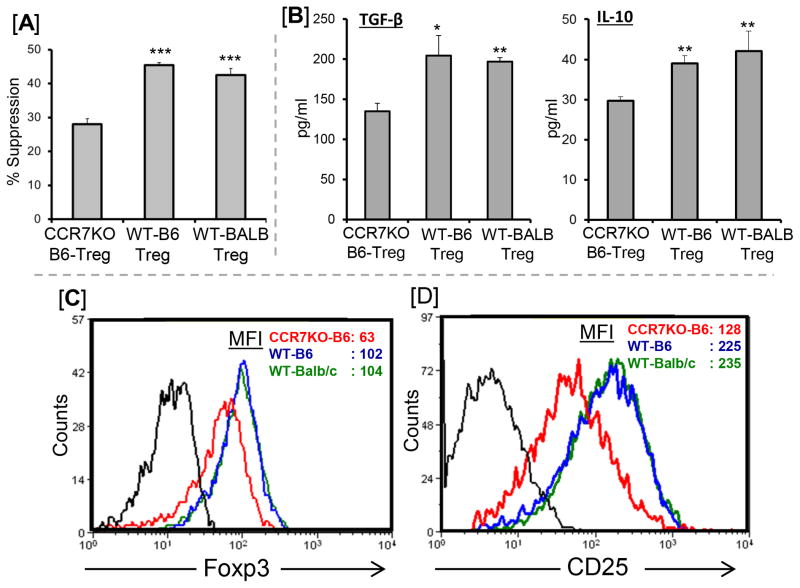
It is still not clear whether, in addition to affecting Treg migration (15, 21), CCR7 expression itself affects Treg suppressive function. Using the in vitro suppression assay, we compared the suppressive function of Tregs isolated from CCR7-KO (C57BL6) and wild type (C57BL6 and BALB/c) mice (Fig 2A). CCR7-KO Tregs demonstrated a 40% reduction in the suppression of anti-CD3 stimulated CD4+ T cell proliferation compared to wild type Tregs (P=0.001). Similarly, culture supernatants from CCR7-KO Tregs showed significantly lower levels of immunoregulatory cytokines IL-10 and TGF-β compared to wild type (C57BL6 and BALB/c) Tregs (Fig 2B). Moreover, a 40–50% decrease in the expression levels of Foxp3 (Fig 2C) and CD25 (Fig 2D) were observed in CCR7-KO Tregs compared to wild type Tregs. Together, these findings suggest that CCR7 expression on Tregs is capable of affecting both their phenotype and suppression potential.
Figure 2. Effect of CCR7 expression on Treg function and phenotype.
(A) Treg suppression function. Naïve wildtype (WT) CD4+ T cells were stimulated with CD3-Ab for 3 days in the presence of different Tregs isolated from CCR7-KO, and WT-C57BL/6 and WT-Balb/c. CCR7-KO Tregs show a 40% reduction in the suppression of CD4+ T cell proliferation compared to WT-Tregs. The activity of Tregs is measured at Treg:Teff cell ratio of 1:2. Proliferation was measured using the BrdU incorporation assay and compared with the proliferative responses of respective CD3-stimulated naive T cell in the absence of Tregs and percent suppression was calculated as described in Materials and Methods. (B) Expression of immunoregulatory cytokines IL-10 and TGF-β at protein levels in the culture supernatants from Treg suppression assays were analyzed using ELISA. (C) Foxp3 and (D) CD25 expressions by CD4+CD25+Foxp3+ Tregs were analyzed by measuring mean fluorescent intensity (MFI) using flow cytometry. Each group consists of three mice, and data from a representative experiment of three performed is shown. p values are calculated using Student’s t test and error bars represent SEM. ***p < 0.001, **p < 0.01, *p < 0.02
CCL21 signaling amplifies Treg expression of CCR7
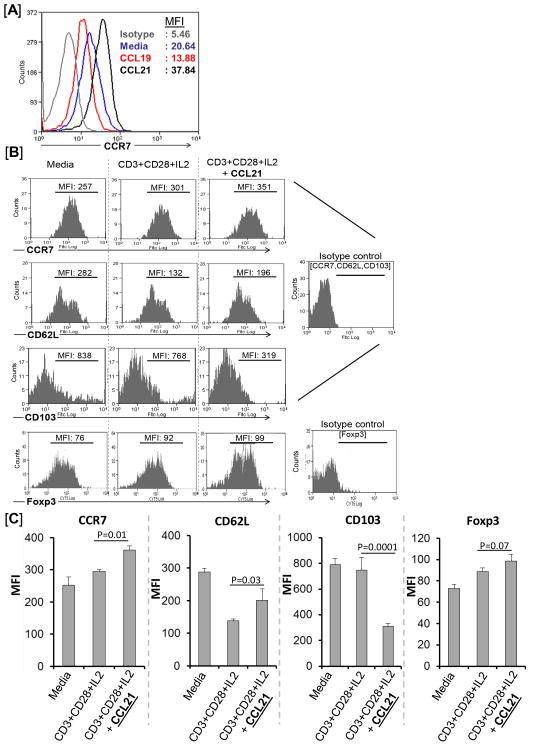
Next, we determined whether naïve Tregs could be induced to express high levels of CCR7, which could in turn enhance both their homing to the reactive draining lymph nodes as well as their suppressive function. Since binding of cytokine or chemokine ligands to their receptors normally leads to cell activation and increased expression of receptors, we first stimulated purified naïve Tregs in vitro with CCR7 ligands for 48 hours to upregulate Treg expression of CCR7 (Fig 3A). CCR7 has two chemokine ligands, CCL19 and CCL21, which are normally expressed in the T cell-rich areas of the lymph nodes (15). In vitro stimulation with CCL21 upregulated Treg expression of CCR7 substantially (~1.5–2 fold; MFI: 38) compared to media only (MFI: 21), while CCL19 stimulation led to down regulation of Treg expression of CCR7 (MFI: 14).
Figure 3. Amplification of Treg expression of homing receptors ex vivo.
(A) Comparative effect of increased CCL19 vs. CCL21 signaling on Treg expression of CCR7. Flow cytometric analysis shows that Tregs cultured for 48h in the presence of CCL21 (black line) but not CCL19 (red line) upregulate CCR7 expression (mean fluorescent intensity, MFI) compared to those in media only (blue line). (B) Representative flow cytometry histograms and (C) cumulative data as bar diagrams show that addition of CCL21 to cytokine cocktail (CD3+CD28+IL2) for ex vivo Treg expansion (incubated for 5 days) upregulates expression of CCR7 and CD62L, and downregulates expression of CD103. Furthermore, CCL21 signaling leads to an increase in Foxp3 expression levels. Data from a representative experiment of three performed is shown.
The application of Tregs as a cellular therapeutic to promote transplant survival requires their isolation and massive in vitro expansion. We thus next investigated if the addition of CCL21 during in vitro Treg expansion could lead to increased expression of the LN homing receptor CCR7 (Fig 3B and 3C). Conditioning media consisting of CD3 agonist antibody in conjunction with IL-2 (T cell growth factor) and CD28 (costimulatory factor) agonist antibody were used to expand Tregs in vitro for 5 days (20), with or without addition of CCL21 (Fig 3B and 3C). Tregs cultured and expanded in the presence of CCL21 expressed increased levels of the LN homing receptors CCR7 and CD62L, and decreased levels of CD103 compared to those Tregs that were cultured in Treg expansion cocktail or media only. Furthermore, the addition of CCL21 to Treg expansion media led to increased expression of Foxp3 by Tregs. However, as shown in the Supplement section, stimulation of CCR7-KO Tregs with CCL21 alone or CCL21 and Treg expansion media did not show any direct effect of CCL21 function on Treg upregulation of CCR7 and Foxp3 expression (Fig S1). Together these findings confirm that addition of the CCR7-ligand CCL21 to Treg expansion media increases Treg activation and expression of CCR7 and CD62L, resulting in an amplified LN homing Treg phenotype (CCR7hiCD62LhiCD103lo).
CCR7-amplified Tregs acquire maximal LN homing in allograft recipients and promote transplant survival
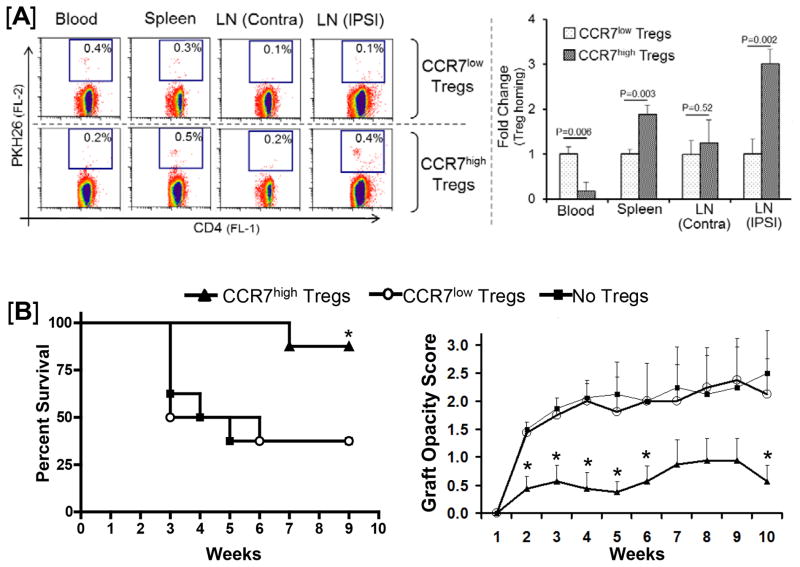
To determine the in vivo function of Tregs amplified for LN homing in corneal transplant hosts, purified Tregs from naïve Balb/c mice were cultured with CCL21 (CCR7hi Tregs) or without CCL21 (CCR7lo Tregs) and then labeled with vital cell tracking dye PKH26. Intravenous infusion of labeled Tregs into corneal allograft recipients was performed 24h after transplantation, a time point based on the observation of considerable numbers of graft-derived APC in the draining LN of recipients 24h post-grafting (16), and our previous work on Treg adoptive transfer in corneal transplant recipients (18). Three mice from each group were sacrificed 48h post Treg infusion to localize Tregs in the LN, spleen and blood, while the remaining mice (n=8–10/group) were used to compare allograft survival up to 8 weeks post-transplantation.
CCR7hi Tregs showed efficient homing to the lymphoid tissues whereas CCR7lo Tregs were primarily retained in the peripheral blood of allograft recipients (Fig 4A). CCR7hi Tregs demonstrated a 4-fold increase in the rate of homing to the reactive ipsilateral draining LN, as compared to CCR7lo Tregs. In addition, the allograft recipients who received CCR7hi Tregs demonstrated a significant increase in graft survival rate and reduced graft opacity scores compared to allograft recipients receiving either CCR7lo Tregs or no Tregs (Fig 4B). The allograft survival rate in recipients receiving CCR7lo Tregs was similar to that of recipients receiving no Tregs, suggesting that CCR7lo Tregs were unable to promote transplant survival. Together, these experiments demonstrate that Tregs which are ex vivo amplified for CCR7 expression acquire maximal homing to the draining LN in allograft recipients and promote transplant survival.
Figure 4. In vivo function of Tregs amplified for LN homing (CCR7hi) in corneal allograft recipients.
CCR7hi and CCR7lo Tregs were generated by culturing Tregs in Treg expansion cocktail (CD3+CD28+IL2) with or without CCL21 stimulation for 5 days. (A) Homing of CCR7hi vs. CCR7lo Tregs to lymphoid tissues. Treg homing analysis in transplant recipients was performed 2 days after adoptive transfer of PKH26-labeled Tregs. Allograft recipients show significantly enhanced LN-homing of CCL21-stimulated CCR7hi expressing Tregs (>3-fold in ipsilateral reactive LN) compared to CCR7lo Tregs. (B) Effect of CCR7hi vs. CCR7lo Tregs on graft survival. Only the group that adoptively receives CCL21-stimulated CCR7hi Tregs showed significant increase in the allograft (B6→BALB/c) survival rate (87%, n=8/group, p=0.022) with significantly lower corneal graft opacity scores; no improvement in graft survival is observed in the recipients which receive CCR7lo expressing Tregs.
DISCUSSION
In the present study we show that Tregs of allograft acceptors but not rejectors colocalize with APC in the paracortical areas of the draining LN and express high levels of CCR7. In addition, our data provide novel evidence that CCR7 expression affects Treg suppressive function, and that increased CCL21 signaling in Tregs can amplify their expression of CCR7 and thus promote their tolerogenic function and promotion of transplant survival.
Previous studies localizing Foxp3+ Tregs in steady state normal (ungrafted) lymphoid tissue have shown that they are evenly distributed throughout the paracortex (22). However, the localization of Tregs in the draining lymphoid tissues of transplant recipients has hitherto not been established. Our data derived from a well characterized model of allotransplantation show the focal co-localization of Tregs with APC in the paracortical region of draining LN of allograft acceptors. In allograft rejectors, in contrast, Tregs remain evenly distributed throughout the paracortex similar to the steady state normal LN (22), and rarely come in contact with APC. Furthermore, Tregs of allograft acceptors express higher levels of LN homing receptors, particularly CCR7, compared to Tregs of rejectors. There is evidence indicating that CCR7 and CD62L work in concert to promote Treg homing to and migration within the LN (8, 13). Conversely, CD103 (integrin αE- E-cadherin-binding) has been implicated in Treg retention in peripheral tissues (10, 23). Together, these findings indicate that Tregs in the LN of allograft acceptors are better able to migrate toward APC, promoting immunoregulatory function at the immune synapse, and that CCR7 could be the most critical homing receptor regulating Treg migration towards APCs in the LN. Furthermore, our data evaluating the function and phenotype of CCR7-KO and wild type Tregs demonstrate that deletion of CCR7 leads to a significant 40% reduction in Treg suppression potential as well as decreased expression of Foxp3 and immunoregulatory cytokines IL-10 and TGF-β compared to wild type Tregs, clearly suggesting that in addition to affecting Treg migration (15, 21), CCR7 expression by Tregs is capable of influencing both their phenotype and suppression potential.
To determine the therapeutic implication of these findings, we next investigated whether naïve Tregs could be induced to express high levels of CCR7, which would in turn enhance both their homing to the reactive draining lymph nodes and their suppressive function. In vitro stimulation of Tregs with the CCR7 ligand CCL21, but not CCL19, led to an upregulation of Treg expression of CCR7. The chemokines CCL19 and CCL21, which are normally expressed in the T cell-rich areas of the lymph nodes (15), have previously been reported to be indistinguishable in their binding affinities for CCR7 and their ability to regulate chemotaxis and migratory speed (24, 25). However, they significantly differ in their regulation of cytoarchitecture following receptor binding; indeed, it has been observed that CCL19, but not CCL21, induces internalization of CCR7 and desensitizes receptor-expressing T cells and mature dendritic cells to a second stimulus of the chemokine (24, 25). Together, these observations corroborate our findings and suggest that CCL21 (but not CCL19) signaling can optimally upregulate Treg expression of CCR7. In addition, our data demonstrate that CCL21 is primarily expressed at high levels in the LN of allograft acceptors, whereas CC19 is highly expressed in the LN of allograft rejectors (Fig S2), which further support our findings showing increased expression of CCR7 by allograft acceptor-Tregs, but not by allograft rejector-Tregs (Fig 1B).
The use of Tregs as a cell-based therapy to promote transplant survival requires their in vitro expansion. We show herein that the addition of CCL21 during in vitro Treg expansion leads to increased expression of the LN homing receptors CCR7 and CD62L. Interestingly, CCL21-signaling decreases the Treg expression of CD103, which has been shown to play a critical role in Treg retention in peripheral tissues, reducing their homing to the lymphoid tissues (10, 26). A previous study has reported that after ex vivo expansion of Tregs, Foxp3 expression and suppressive activity are maintained only in Tregs that express LN-homing CCR7 and CD62L molecules, data that are in accord with our present findings (27). Finally, the functional relevance of ex vivo CCR7-amplified Tregs in promoting transplant survival is fundamentally confirmed by our novel finding that adoptive transfer of CCR7hi Tregs to allograft recipients results in markedly increased homing to the reactive draining LN, which is paralleled by an increased rate of allograft survival. In contrast, CCR7lo Tregs could not preferentially home to the lymphoid tissues, and the allograft survival rate in recipients receiving CCR7lo Tregs was similar to that of recipients receiving no Tregs, suggesting that CCR7lo Tregs are unable to promote transplant survival. A small number of both CCR7hi and CCR7lo expressing Tregs were also detected in transplanted corneas but not the contralateral ungrafted cornea (Fig S3). These data corroborate our previous findings (18) showing the presence of Tregs in the cornea of both allograft acceptors and allograft rejectors, and the draining LN as the primary site where Tregs exert their suppressive function and promote allograft survival.
In summary, our study provides new insights on the role of Tregs and their mechanism of action in the suppression of alloimmunity in transplantation. CCR7hi Tregs comprise the most functional subset of Tregs in the lymphoid tissues where they interact with incoming graft-derived APC and regulate induction of alloimmune responses. By guiding a variety of immune cells to and within the lymphoid tissues, CCR7 thus contributes to both immunity and tolerance (15, 28) since this receptor is also involved in lymph-node homing of naive T cells and tissue-derived dendritic cells. The novel findings presented herein demonstrate that in addition to its role in homing, CCR7 expression by Tregs also regulates their suppressive function, suggesting that CCR7-associated impaired migration has functional implications related to allograft rejection. Further work is needed to understand the underlying mechanisms of how the CCR7-signaling pathway in Tregs is linked to Foxp3 and CD25 expression. Importantly, the present study describes a novel strategy of enhancing CCL21-mediated CCR7-signaling in Tregs during ex vivo expansion in order to augment their lymph node-homing characteristics (CCR7hiCD62Lhi CD103lo) without losing their suppressive phenotype. These findings may have important implications for future translational studies utilizing Tregs as a potential means of promoting allograft survival and treating other T cell–mediated diseases.
Supplementary Material
Footnotes
This work was supported in part by grants from Eye Bank Association of America to S.K.C., and NIH (NEI-12963) to R.D.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-) evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 4.Thomson AW, Turnquist HR, Zahorchak AF, Raimondi G. Tolerogenic dendritic cell-regulatory T-cell interaction and the promotion of transplant tolerance. Transplantation. 2009;87:S86–90. doi: 10.1097/TP.0b013e3181a2dcec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 7.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huehn J, Hamann A. Homing to suppress: address codes for Treg migration. Trends Immunol. 2005;26:632–636. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schön MP, Scheffold A, Lowe JB, Hamann A, Syrbe U, Huehn J. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Llodra J, Ding Y, Lira SA, Krieger NR, Bromberg JS. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 12.Ueha S, Yoneyama H, Hontsu S, Kurachi M, Kitabatake M, Abe J, Yoshie O, Shibayama S, Sugiyama T, Matsushima K. CCR7 mediates the migration of Foxp3+regulatory T cells to the paracortical areas of peripheral lymph nodes through high endothelial venules. J Leukoc Biol. 2007;82:1230–1238. doi: 10.1189/jlb.0906574. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Bromberg JS. T regulatory cells and migration. Am J Transplant. 2006;6:1518–1523. doi: 10.1111/j.1600-6143.2006.01372.x. [DOI] [PubMed] [Google Scholar]
- 14.Worbs T, Mempel TR, Bölter J, von Andrian UH, Förster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005;174:6577–6581. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 20.Ring S, Thome M, Pretsch L, Enk AH, Mahnke KK. Expanded murine regulatory T cells: analysis of phenotype and function in contact hypersensitivity reactions. J Immunol Methods. 2007;326:10–21. doi: 10.1016/j.jim.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hontsu S, Yoneyama H, Ueha S, Terashima Y, Kitabatake M, Nakano A, Ito T, Kimura H, Matsushima K. Visualization of naturally occurring Foxp3+ regulatory T cells in normal and tumor-bearing mice. Int Immunopharmacol. 2004;4:1785–1793. doi: 10.1016/j.intimp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, Schmidt CA, Bräuer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive-and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardi G, Lipp M, Baggiolini M, Loetscher P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur J Immunol. 2001;31:3291–3297. doi: 10.1002/1521-4141(200111)31:11<3291::aid-immu3291>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem. 2007;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc Natl Acad Sci U S A. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 28.Jin Y, Chauhan SK, Saban DR, Dana R. Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation. Invest Ophthalmol Vis Sci. 2010;51:816–821. doi: 10.1167/iovs.09-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.