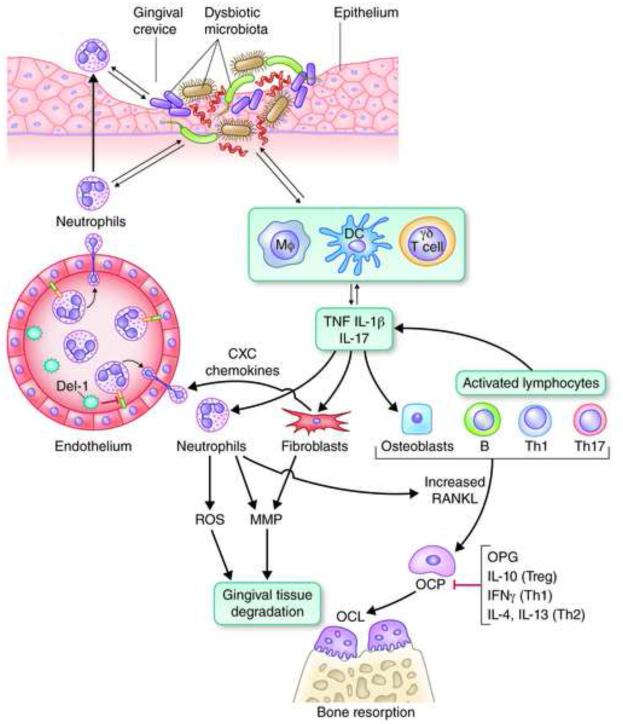
Fig. 2. Inflammatory mechanisms leading to bone loss in periodontitis.
Recruited neutrophils to the gingival crevice fail to control a dysbiotic microbiota, which can thus invade the connective tissue and interact with additional immune cell types, such as macrophages (Mφ), dendritic cells (DC), and γδ T cells, a subset of innate-like lymphocytes. These cells produce proinflammatory mediators (such as the bone-resorptive cytokines TNF, IL-1β, and IL-17) and also regulate the development of Th cell types, which also contribute to and exacerbate the inflammatory response. IL-17, a signature cytokine of Th17 (though also produced by innate cell sources), acts on innate immune and connective tissue cell types, such as neutrophils, fibroblasts, and osteoblasts. Through these interactions, IL-17 induces the production of CXC chemokines (which recruit neutrophils in a Del-1–dependent manner), matrix metalloproteinases (MMPs) and other tissue-destructive molecules (e.g., ROS), as well as osteoblast expression of RANKL, which drives the maturation of osteoclast precursors (OCP). Activated lymphocytes (B and T cells, specifically Th1 and Th17) play a major role in pathologic bone resorption through the same RANKL-dependent mechanism, whereas osteoprotegerin (OPG) is a soluble decoy receptor that inhibits the interaction of RANKL with its functional receptor (RANK) on OCP. The RANKL/OPG ratio increases with increasing inflammatory activity. Activated neutrophils express membrane-bound RANKL and could directly stimulate osteoclastogenesis if they are within sufficient proximity to the bone. The anti-inflammatory cytokine IL-10 (produced by Tregs), as well as IFNγ (produced by Th1 cells) and IL-4 plus IL-13 (produced by Th2 cells) can suppress osteoclastogenesis. The innate-adaptive cell interplay is considerably more complex than depicted here but serves to illustrate major destructive mechanisms operating in the context of unresolved periodontal infection and inflammation.