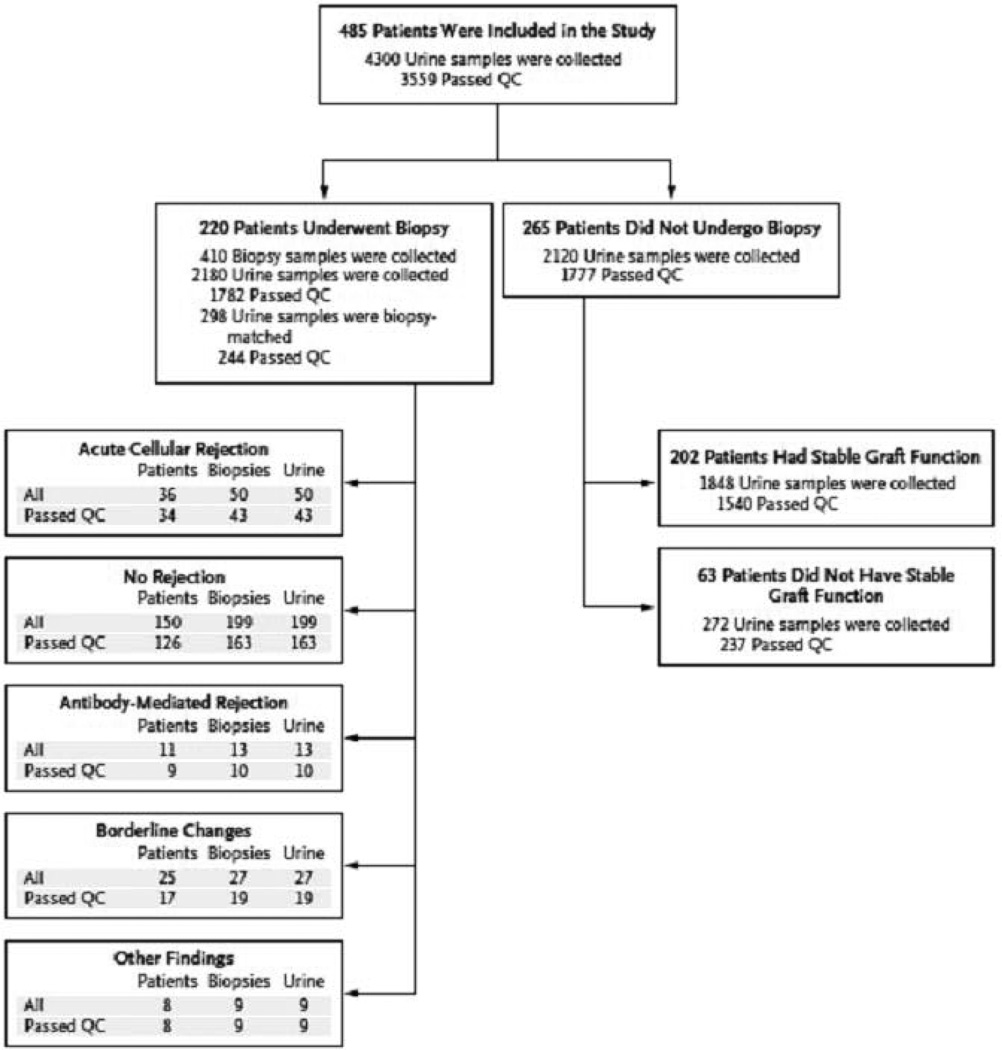
Fig. 2. Patients, biopsy results, and urine samples.
A total of 4300 urine samples were collected from 485 kidney allograft patients for urinary-cell messenger RNA (mRNA) profiling on days 3, 7, 15, and 30, in months 2, 3, 4, 5, 6, 9, and 12 after transplantation, and at the time of any kidney-allograft biopsy and 2 weeks thereafter. Of the 4300 urine specimens, 3559 were classified as passing quality control (QC) and 741 were classified as not passing. A total of 220 patients underwent 410 kidney-allograft biopsies, and 265 did not undergo biopsy. The numbers of patients with biopsy-matched urine samples (urine samples that were collected from 3 days before to 1 day after biopsy and that passed QC) are shown for patients with acute cellular rejection (defined as Banff grade IA or higher), for those without any rejection features in the biopsy sample, for those with acute antibody-mediated rejection, for those with borderline changes, and for those with other biopsy findings. The number of patients listed under different diagnostic categories exceeds the 220 patients who underwent biopsy because several patients had multiple biopsies with different diagnoses. Among the 265 patients who did not undergo biopsy, 202 met the criteria for stable graft function, of whom 201 had urine samples that passed QC (from Suthanthiran et al. N Engl J Med 2013, with permission).