Figure 4. Biochemical evidence for incomplete execution of apoptotic cell death following neonatal HI.
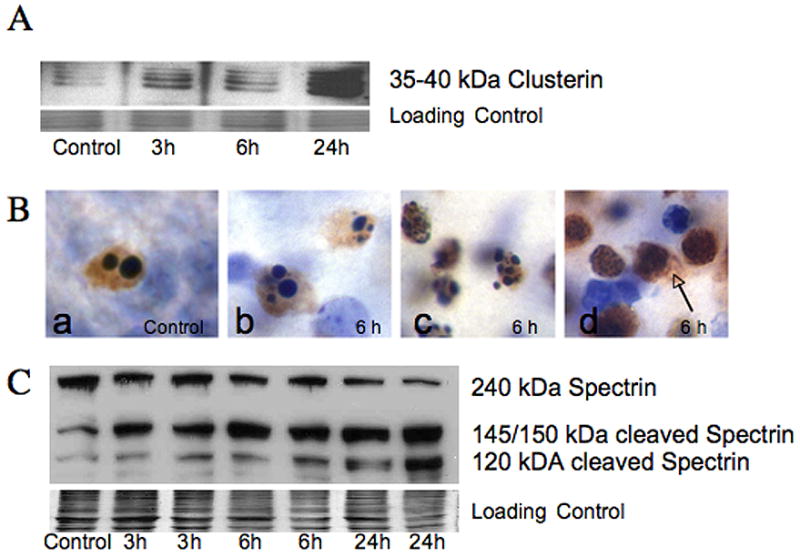
A. Clusterin. a multimeric 35–40 kDa glycoprotein, thought to be a marker for excitotoxic neurodegeneration (Han et al., 2001) is present in the soluble fraction from ipsilateral forebrain as early as 3 hours, and is markedly elevated at 24 hours following neonatal HI simultaneous with increased p17 caspase 3 fragment (Figure 1C). The commassie stained gel is shown as the loading control. B. Histochemical expression of the 17 kDa active fragment of caspase-3 is found in dying striatal neurons following neonatal HI (brown immunoreactivity, cells counter-stained with CV). The 17 kDa isoform of caspase-3 is present in cells dying with a variety of nuclear morphologies. Cells dying from programmed cell death in control tissue (Ba) as well as cells with classic apoptotic morphology in injured striatum (Bb) are immunoreactive for 17 kDa caspase-3 as expected. Other striatal neurons with immunoreactivity for 17 kDa caspase-3 have nuclear morphologies not consistent with classic apoptosis, but rather show incomplete nuclear fragmentation (Bc) and some have minimal evidence of chromatin condensation (Bd). Spillage of p17 caspase 3 into extracellular space is seen in cells with the least evidence of organized chromatin condensation (arrow) presumably due to disruption of cell membrane. C. Immunoblot showing cleavage of 240 kDa full length spectrin, an important cytoskeletal protein, is shown. Expression of the 145/150 kDa spectrin fragment, produced by excitotoxic- calpain mediated fragmentation occurs as early as 3 hours following HI while expression of the 120 kDa caspase-cleaved fragment appears at 3 and 6 hours but in much more abundance at 24 hours after HI. The commassie stained gel is shown as the loading control.
