Abstract
We assessed ferumoxytol-enhanced brain MRI to identify monocyte/macrophage accumulation in HIV-associated neurocognitive disorder (HAND). Four HIV-infected subjects with undetectable HIV RNA levels on antiretroviral therapy, HIV DNA level in CD14+ cells ≥ 10 copies/106 cells, and cognitive impairment underwent ferumoxtyol-enhanced brain MRI. On post-ferumoxytol susceptibility-weighted images, all HIV-infected subjects demonstrated a diffuse “tram track” appearance in the perivascular regions of cortical and deep white matter vessels suggesting ferumoxytol uptake in monocytes/macrophages. This finding was not present in a HIV-seronegative control. While ferumoxytol may have potential as an imaging biomarker for monocytes/macrophage accumulation in patients with HAND, future study is needed.
Keywords: HIV, HIV dementia, MRI, ferumoxytol
INTRODUCTION
HIV-associated neurocognitive disorder (HAND) is present in more than 50% of HIV-infected individuals who are otherwise doing well on combination antiretroviral therapy (cART).1 Monocytes/macrophages (M/MΦ) are believed to play a critical role in the pathogenesis of HAND. This model suggests that HIV-infected M/MΦ cross the blood brain barrier and precipitate an inflammatory cascade particularly in the perivascular regions of the brain, leading to indirect neuronal damage and ultimately to cognitive dysfunction.2 This hypothesis is supported by pathologic studies that have demonstrated continued predominantly perivascular accumulation of macrophages within the brain in autopsy series of HIV-infected individuals who died while on cART.2, 3
Currently, no neuroimaging modality exists that can define the extent of brain M/MΦ accumulation in HAND either as a clinical diagnostic tool or to assist in defining objective improvement in clinical trials addressing HAND. Ferumoxtyol is an ultra-small iron oxide MRI contrast agent avidly taken up by circulating M/MΦ.4 It is currently FDA approved for the treatment of iron deficiency anemia in patients with chronic kidney disease. Iron oxide compounds such as bromodeoxyuridine have been successfully used in simian immunodeficiency virus (SIV) disease to define the role of macrophages in the development of encephalitis in this animal model.5 We therefore hypothesized that ferumoxytol may have a potential role in neuroimaging in humans and should demonstrate perivascular uptake in tagged M/MΦ on conventional T1- and T2- weighted images in individuals with HAND. We report in this pilot study, the first investigation of the safety, tolerability, and neuroimaging characteristics of ferumoxytol-enhanced brain MRI in HAND.
METHODS
Study population
We recruited HIV-infected subjects with undetectable plasma HIV RNA levels who received uninterrupted cART for ≥ 6 months, had a peripheral blood mononuclear cell (PBMC) HIV DNA level in CD14+ (M/MΦ) cells ≥ 10 copies/106 CD14+ cells,6 and demonstrated cognitive impairment on neurocognitive testing. Cognitive impairment was defined as a composite z-score of global cognitive impairment (NPZglobal) ≤ - 0.5 calculated as the arithmetic mean of age- and education-adjusted z-scores on 14 neurocognitive test variables or a composite z-score involving a cognitive domain typically impacted by HIV (e.g., psychomotor speed, attention and concentration, memory and learning, information processing speed, executive function). PBMC HIV DNA level assessed specifically in CD14+ (M/MΦ) cells ≥ 10 copies/106 CD14+ cells was also a criteria for inclusion because our research group has consistently demonstrated a robust association of PBMC HIV DNA in CD14+ cells with HAND.6 Exclusion criteria included subjects receiving treatment for an acute AIDS-defining illness, current active substance or alcohol dependence, a known hypersensitivity reaction to iron, history of an iron overload syndrome, receiving frequent blood transfusions, taking oral iron supplementation, history of insulin-dependent diabetes mellitus, traumatic brain injury, epilepsy requiring treatment with an antiepileptic, learning disability, AST or ALT greater than twice the upper limits of normal, elevated iron levels, hematocrit > 52% on pre-entry baseline laboratory safety assessment, and a positive urine toxicology screen on the day of neurocognitive testing. U.S. FDA (IND 114,001), Western Institutional Review Board and University of Hawaii Committee on Human Studies approved this study (ClinicalTrials.gov NCT01665846).
MRI Protocol
Brain MRIs were performed on a 3.0 Tesla Philips Achieva scanner. Axial 2D T1-weighted spin echo image (TR/TE 900/10 ms; FA 90°; FOV 240×240 mm2; matrix 268x268; slice thickness 2 mm; interslice gap 0 mm; number of slices 44), T2-weighted turbo spin echo image (TR/TE 9000/93 ms; FA 90°; FO V 240×240 mm2; matrix 256×256; slice thickness 2 mm; interslice gap 0 mm; TSE factor 9), dynamic T2*-weighted image using gradient echo echo-planar sequences (TR/TE 1500/20 ms; FA 45°; FOV 192×192 mm; matrix 64×64; slice thickness 3 mm; interslice gap 0.9 mm; number of slices 27), and susceptibility-weighted imaging (SWI) 3D venous BOLD image (TR/TE 32.12/42.35 ms; FOV 240×240 mm2; matrix 560×560; slice thickness 2.4 mm; interslice gap 1.2 mm; GR 15; number of slices 72) were obtained immediately prior and 48 hours after IV ferumoxytol infusion (dose of 4 mg Fe/kg up to a maximum of 510 mg of elemental iron).
Control subject
Investigator's (BEH) personal database was used to identify HIV-seronegative control subjects who had brain imaging performed before and 48 hours after IV ferumoxytol infusion.
RESULTS
Clinical characteristics of HIV-infected subjects
Four subjects (all male; mean age 60.25 ± 5.85 years, range: 54-67; mean years since first diagnosis 18.50 ± 6.45 years, range: 13-27; mean current CD4 count 631± 128 cells/mm3, range: 468-742; mean nadir CD4 count 158 ± 64 cells/mm3, range: 93-245; none co-infected with hepatitis C virus (HCV)) were recruited. Mean PBMC HIV DNA in CD14+ cells was 576 ± 749 copies/106 CD14+ cells (range: 10-1675). Mean hematocrit was 44% (range: 39-48) and mean ferritin 358 ng/mL (range 41-1190). Mean score on Beck Depression Inventory was 6 ± 1.15 (range: 5-7). Three subjects had global cognitive impairment (mean NPZglobal = -1.33 ± 0.60, range -1.99 to -0.80) while one subject had impairment in memory/learning efficiency (NPZlrn_mem = -1.04) and normal global cognitive performance (NPZglobal = 0.1). One subject had a history of coronary artery disease and stroke. All subjects had hyperlipidemia, two had hypertension, one used tobacco, and one subject was overweight. The infusion was well tolerated and there were no adverse events based on Division of AIDS Adverse Events grading table during or one-hour following ferumoxytol administration or at one-month follow-up.
Imaging findings of HIV-infected subjects
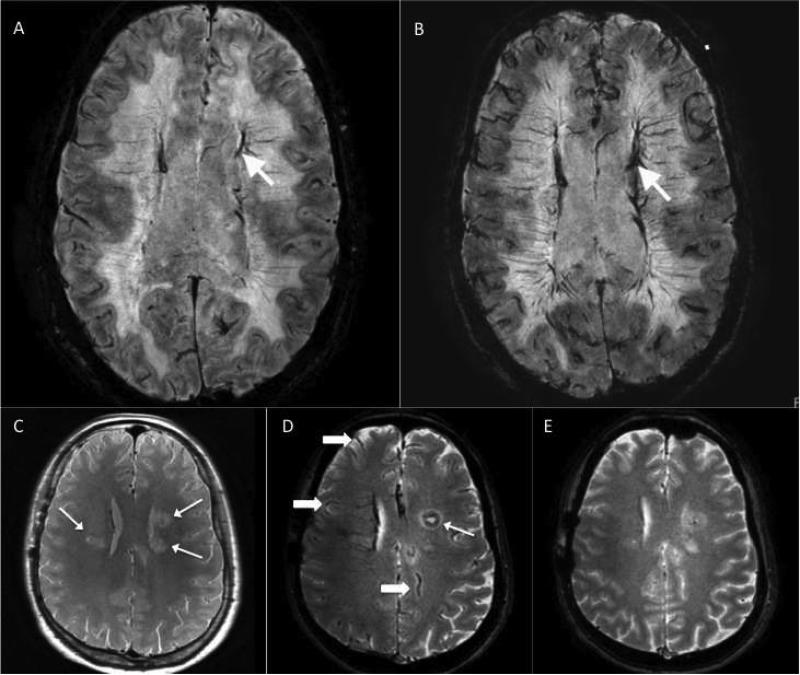
On SWI performed 48 hours after ferumoxytol infusion, all subjects demonstrated diffuse increased vascular caliber with a “tram track” appearance suggesting perivascular ferumoxytol uptake in M/MΦ in or adjacent to the arterial and venous intracranial vessel walls of the peripheral cortical and deep white matter vasculature (Figure). Small residual intravascular contrast was present in all post-ferumoxytol T1-weighted sequence brain MRIs (not shown in Figure).
Figure. Axial susceptibility-weighted brain MRI (A and B) in a HIV-infected study subject.
Pre-contrast image (A) shows no focal abnormality; vessels show normal hypointensity before contrast (arrow). Post-contrast image obtained 48 hours post-ferumoxytol infusion (B) suggests areas of increased vascular caliber with a “tram track” appearance that might reflect perivascular ferumoxytol uptake in monocytes/macrophages in or adjacent to some of the arterial and venous intracranial vessel walls (arrow). These (A and B) are representative images of all study subjects. Axial T2* gradient recalled echo brain MRI (C, D, and E) in the control HIV-seronegative subject with glioblastoma multiforme (GBM). Pre-contrast image (C) shows multicentric nodules consistent with known glioblastoma multiforme. Post-contrast image obtained 24 hours post-ferumoxytol infusion demonstrates minimal ferumoxytol enhancement within a GBM nodule in the left frontal lobe (thin arrow) and decreased signal intensity from intravascular ferumoxytol (thick arrows). There is near complete resolution of intravascular ferumoxytol 48 hours post-ferumoxytol infusion. The abnormalities demonstrated in our HIV-infected subjects were not present.
No subject demonstrated abnormal parenchymal enhancement on any sequence. Two subjects had generalized cerebral atrophy. One subject had diffuse symmetric leukoaraiosis, and another subject had focal encephalomalacia involving the left periventricular white matter and basal ganglia consistent with remote infarction.
HIV-seronegative control subject
One HIV-seronegative control subject was identified who had imaging performed before and 24 and 48 hours after IV ferumoxytol infusion. The subject was a 48-year old male with biopsy-proven glioblastoma multiforme (GBM). Brain MRI was performed on the same scanner and with same protocol as HIV-seropositive subjects except that a gradient recalled echo (GRE) sequence (TR 750-2969 ms; T2* GRE TE 16-23 ms; FA 10-18°; matrix 256×256; slice t hickness 3-4 mm) was performed instead of SWI. Pre-ferumoxtyol GRE sequences revealed multicentric nodules in the frontal lobes consistent with subject's known GBM history (Figure). There was minimal ferumoxytol enhancement in one nodule in the left frontal lobe in addition to decreased intravascular signal from ferumoxtyol on repeat imaging 24 hour after ferumoxytol infusion (Figure). At 48 hours post-ferumoxytol infusion, there was near complete resolution of intravascular enhancement (Figure). The diffuse increase in vessel caliber and perivascular “tram track” appearance demonstrated on post-ferumoxytol images in HIV-infected subjects was not evident.
DISCUSSION
Our findings suggest that ferumoxytol is safe and well tolerated in HIV-infected individuals and may have a potential role as an imaging biomarker in HAND.
In our study, four treated HIV-infected subjects with an undetectable plasma HIV RNA viral load but detectable levels of PBMC HIV DNA in M/MΦ underwent ferumoxytol-enhanced brain MRI. All HIV-infected subjects demonstrated diffuse linear “tram track” hyperintensities along the walls or perivascular spaces of the intracranial vasculature on ferumoxytol-enhanced SWI suggesting abnormal ferumoxytol uptake by M/MΦ in these areas which was not evident in our HIV-seronegative control (Figure). Our findings are clinically relevant given that the failure to eradicate sequestered HIV reservoirs in M/MΦ has emerged as one fundamental barrier to clearance of HIV in individuals treated with cART.7 Standard therapies do not target HIV-infected peripheral monocytes, and it is believed that this viral reservoir traffics across the blood-brain barrier causing indirect injury to the brain via persistent immune activation and inflammation.8 Prior studies have demonstrated that proviral HIV DNA in M/MΦ remains detectable despite treatment with cART and the burden of virus is associated with not only neurocognitive impairment but also HIV disease progression and the development of AIDS.6,9 While our results are preliminary, it is intriguing to hypothesize that the perivascular “tram track” hyperintensities represent the radiologic correlate of perivascular macrophages that have been demonstrated in histologic studies of HIV encephalitis which are trafficking across the blood-brain barrier. 3
Positron electron tomography (PET) has also been used to study the role of M/MΦ in the pathogenesis of HAND using radioligands, such as [11C]-PK11195, which target activated M/MΦ.10, 11 In a small study of five subjects, Hammoud et al.10 demonstrated increased [11C]-PK11195 uptake in the cortex and basal ganglia in three HIV-infected individuals with severe cognitive impairment compared to HIV-seronegative controls. Another PET study using [11C]-PK11195 by Wiley et al., however, demonstrated that there were no differences in uptake in 12 HIV-infected subjects with mild cognitive impairment compared to 5 HIV-seronegative controls.11 Therefore, while [11C]-PK11195 uptake using PET is increased in HIV-infected individuals with severe cognitive impairment, it appears insensitive to detecting those individuals with milder degrees of cognitive impairment, which account for a majority of HIV-infected patients in the era of cART. A more recent study by Garvey et al.12 found that no differences in activated M/MΦ in the brain as measured by [11C]-PK11195 were found between 8 HIV-infected individuals with acute HCV coinfection compared to 8 HIV-monoinfected individuals. Garvey et al.12 hypothesized that further recruitment of activated M/MΦ may take place only during the later stages of chronic HCV co-infection to account for the increased cognitive impairment in HCV-HIV coinfected individuals. None of our subjects were co-infected with HCV. In our study, 3 out of the 4 HIV-infected subjects had mild cognitive impairment; however further study is needed to determine if there are differences in ferumoxytol enhancement on brain MRI performed in HIV-infected individuals with and without mild cognitive impairment.
Ferumoxytol-enhanced MRI for assessing intracranial enhancement in HAND appears feasible and safe in our exploratory study. Although post-marketing experience with ferumoxytol administered for iron-deficiency anemia has reported a small risk of serious hypersensitivity reactions (0.2%) and clinically significant hypotension (1.9%),13 it was reassuring that no adverse events occurred in our study.
While intriguing, our study has limitations, and the results should be interpreted with caution. First, we recognize only one control subject was obtained and that the control subject in our study had an underlying GBM confounding the MRI appearance. Nonetheless, the abnormalities in our control subject were localized. The diffuse increase in vessel caliber and perivascular “tram track” appearance demonstrated on post-ferumoxytol images in our HIV-infected subjects should not be seen in individuals with GBM,14 which is why this subject was selected as a control. A future study is required to confirm our findings comparing HIV-infected individuals with cognitive impairment to controls, which would include HIV-infected and HIV-seronegative subjects without cognitive impairment on neurocognitive testing and without underlying comorbidities that may confound interpretation of ferumoxtyol-enhanced neuroimaging. Second, although our HIV-infected subjects had undetectable plasma HIV RNA levels, cerebrospinal fluid (CSF) HIV RNA levels were not obtained, and the possible contribution of HIV escape in CSF despite viral control in plasma to the ferumoxytol-enhanced MRI appearance cannot be excluded. Third, there are differences in the MRI protocol used to detect ferumoxytol enhancement in the HIV-infected cases (e.g., SWI) and control (T2* GRE). While SWI and T2* GRE are both sensitive to the magnetic susceptibility contrast provided by ferumoxytol, this variation may account for some of the differences observed between cases and control. Fourth, in our HIV-infected subjects, intravascular contrast was still visible on T1-weighted sequences (not shown in Figure), and we are unable to exclude confounding from persistent intravascular enhancement. Given that the half-life of ferumoxytol is approximately 14 hours,4 future studies involving HIV-infected subjects should include post-ferumoxytol brain imaging at 72 hours. Finally, dose optimization for use of ferumoxytol for neuroimaging in individuals with HAND was not possible in this small group of subjects. While this study used a dose of 4 mg/kg up to a maximum dose of 510 mg of elemental iron, other studies have used doses of 1-6 mg/kg.4 That said, a 510 mg dose is the maximum FDA-approved therapeutic iron replacement dose for renal failure patients with iron deficiency. In addition, a recent study suggests that ferumoxytol doses < 2 mg/kg are ineffective while a 510 mg dose is sufficient for imaging central nervous system pathology.15
In conclusion, ferumoxytol was safely administered to and well tolerated in HIV-infected patients in our preliminary study. While ferumoxtyol may have potential as an imaging biomarker for M/MΦ in patients with HAND, future study is needed.
Acknowledgement
We thank our study participants and community physicians for their roles in this study.
Dr. Nakamoto has received research support from NIH (U54MD007584). Dr. Nakamoto declares that there are no conflicts of interest.
Dr. Shikuma has received research support from NIH (U54MD007584, U54NS43049, P20RR011091, and R01HL095135) Pfizer, Merck, and Gilead Pharmaceuticals, and has served on an advisory board for Glaxo Smith Kline. Dr. Shikuma declares that there are no conflicts of interest.
Dr. Neuwelt has received research support from NIH (R01 CA137488, R01 NS044687, R01 NS053468). Dr. Neuwelt declares that there are no conflicts of interest.
Dr. Shiramizu has received research support from NIH (R01NS053345, U54MD007584, U54NS43049). Dr. Shiramizu declares that there are no conflicts of interest.
Dr. Chow has received research support from NIH (K23 HL088981). Dr. Chow declares that there are no conflicts of interest.
Dr. Kallianpur has received research support from NIH (U54MD007584, U19MH081835, U54MD007584). Dr. Kallianpur declares that there are no conflicts of interest.
Footnotes
Statistics performed by: Beau Nakamoto, University of Hawaii, Honolulu, HI, USA
Author contributions:
Beau K. Nakamoto: study concept and design, acquisition of data, study supervision, statistical analysis, analysis and interpretation, and drafting/revising the manuscript
Cecilia M. Shikuma: study concept and design, acquisition of data, study supervision, statistical analysis, analysis and interpretation, and drafting/revising the manuscript
Debra Ogata-Arakaki: study concept and design, acquisition of data, study supervision, and drafting/revising the manuscript
Tracie Umaki: study concept and design, acquisition of data, study supervision, and drafting/revising the manuscript
Edward A. Neuwelt: study concept and design, analysis and interpretation, and drafting/revising the manuscript
Bruce T. Shiramizu: study concept and design, acquisition of data, contribution of vital reagents/tools/patients, analysis and interpretation, and drafting/revising the manuscript
Dominic C. Chow: study concept and design, analysis and interpretation, and drafting/revising the manuscript
Nisha I. Parikh: study concept and design, analysis and interpretation, and drafting/revising the manuscript
Kalpana J. Kallianpur: study concept and design, analysis and interpretation, and drafting/revising the manuscript
Bronwyn E. Hamilton: study concept and design, acquisition of data, analysis and interpretation, and drafting/revising the manuscript
Conflicts of Interest:
Mrs. Ogata-Arakaki declares that she has no conflict of interest.
Dr. Umaki declares that she has no conflict of interest.
Dr. Parikh declares that she has no conflict of interest.
Dr. Hamilton declares that she has no conflict of interest.
REFERENCES
- 1.Heaton RK, Clifford DB, Franklin DR, Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Palacio M, Alvarez S, Munoz-Fernandez MA. HIV-1 infection and neurocognitive impairment in the current era. Rev Med Virol. 2012;22:33–45. doi: 10.1002/rmv.711. [DOI] [PubMed] [Google Scholar]
- 3.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. Journal of neuropathology and experimental neurology. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 4.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney international. 2009;75:465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soulas C, Conerly C, Kim WK, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiramizu B, Ananworanich J, Chalermchai T, et al. Failure to clear intramonocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. Journal of neurovirology. 2012;18:69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze zur Wiesch J, van Lunzen J. Hide and seek... Can we eradicate HIV by treatment intensification? The Journal of infectious diseases. 2011;203:894–897. doi: 10.1093/infdis/jiq150. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 9.Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 10.Hammoud DA, Endres CJ, Chander AR, et al. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. Journal of neurovirology. 2005;11:346–355. doi: 10.1080/13550280500187351. [DOI] [PubMed] [Google Scholar]
- 11.Wiley CA, Lopresti BJ, Becker JT, et al. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. Journal of neurovirology. 2006;12:262–271. doi: 10.1080/13550280600873868. [DOI] [PubMed] [Google Scholar]
- 12.Garvey LJ, Pavese N, Ramlackhansingh A, et al. Acute HCV/HIV coinfection is associated with cognitive dysfunction and cerebral metabolite disturbance, but not increased microglial cell activation. PLoS One. 2012;7:e38980. doi: 10.1371/journal.pone.0038980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AMGA . Feraheme (ferumoxytol) Injection: Highlights of Prescribing Information. AMGA Pharmaceuticals, Inc.; Lexington, MA: http://wwwaccessdatafdagov/drugsatfda_docs/label/2009/022180lblpdf. [Google Scholar]
- 14.Muldoon LL, Alvarez JI, Begley DJ, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varallyay CG, Nesbit E, Fu R, et al. High-resolution steady-state cerebral blood volume maps in patients with central nervous system neoplasms using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J Cereb Blood Flow Metab. 2013;33:780–786. doi: 10.1038/jcbfm.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]