Abstract
Considerable research indicates that depressed individuals have better memory for negative material than do nondepressed individuals, and that this bias is associated with differential patterns of neural activation. It is not known, however, whether these aberrant activation patterns predict illness course. Using functional neuroimaging, we examined whether change in depressive symptoms is predicted by baseline patterns of neural activation that underlie negative memory biases in Major Depressive Disorder (MDD). Depressed participants viewed negative and neutral pictures during functional magnetic resonance imaging at baseline and completed an incidental memory task for these pictures one week later. Depression severity was assessed by administering the Beck Depression Inventory (BDI) both at baseline (Time 1) and at Time 2, an average of 18 months later. Contrast maps of activation for subsequently remembered negative versus subsequently remembered neutral pictures were regressed against change in BDI scores between Time 1 and Time 2, controlling for initial symptom severity. Results from this analysis revealed no associations between memory sensitivity for negative stimuli and symptom change. In contrast, whole brain analyses revealed significant positive associations between within-subject changes in depressive symptoms and baseline neural activation to successfully recalled negative pictures in the posterior cingulate cortex and medial prefrontal cortex. These findings indicate that neural activation in cortical midline regions is a better predictor of long-term symptomatic outcome than is memory sensitivity for negative material.
Keywords: posterior cingulate, depression, mri, amygdala, prefrontal cortex, symptom prediction
Introduction
Identifying cognitive and neural factors that significantly affect the course of Major Depressive Disorder (MDD) is a clinically relevant and important challenge for behavioral and neuroimaging studies. Considerable research documents that one of the most robust and consistent biases in cognitive processing in MDD involves the preferential recall of negative over positive material [1–3]. Lesion and functional neuroimaging studies show the amygdala is specifically involved in the encoding and retrieval of emotional material [4] by exerting a bottom-up influence on brain regions such as the hippocampus [5]. Neuroimaging studies designed to elucidate the neural underpinnings of negative memory biases in depression have therefore focused on activations in this region. In the first such study, Ramel et al. [6] found that bilateral amygdala response during the encoding of emotional material predicted increased recall of negative (but not of positive) words in formerly depressed participants in whom a sad mood was induced. Similarly, Hamilton and Gotlib [7] documented that increased memory sensitivity for negative stimuli in currently depressed individuals was associated with greater activity in the right amygdala during the successful encoding of this material.
In the present study, we extended Hamilton and Gotlib’s findings by examining, in the same sample of depressed participants, whether the neural abnormalities documented at Time 1 (T1) are useful in predicting changes in depressive symptoms at an 18-month follow-up assessment (T2). Given Hamilton and Gotlib’s results, combined with evidence that greater negative processing biases predict worsening of depressive symptoms [8, 9], we hypothesized that greater amygdala activation during the initial encoding of subsequently remembered negative stimuli would predict more severe depressive symptoms at follow-up. In addition, we conducted whole brain analyses to examine whether activation in other regions that have been associated with aberrant emotion processing in depression [e.g., regions of the default mode and salience networks; 10, 11] are associated with subsequent worsening of depressive symptoms.
Methods
Participants
The study was approved by Stanford University’s institutional review board, and each participant provided written informed consent. Participants were recruited through advertisements posted in numerous locations (e.g., internet bulletin boards, university kiosks, supermarkets, etc.). The Structured Clinical Interview for the DSM-IV [SCID; 12] was administered to all participants to assess current and lifetime diagnoses for anxiety, mood disorders, psychotic symptoms, alcohol and substance use, somatoform, and eating disorders. Medicated subjects were excluded if antidepressant dosage was not maintained 1 month prior to scanning. Severity of depressive symptoms was assessed in all participants at T1 using the Beck Depression Inventory–II [BDI; 13] and again at T2, an average of 18.7 ± 3.5 months later. Informed consent was obtained from each participant, and all participants were paid $25/hour. Of the 14 depressed individuals included in Gotlib and Hamilton’s original investigation, nine participated in this follow-up study. Details are below.
Picture Encoding Task
At T1, participants viewed stimuli, selected from the International Affective Picture System [IAPS; 14] through a projector-directed mirror. Each picture was presented for 2000 ms, followed by ratings for picture intensity and valence. Responses were made with a four-button fMRI response box. For the remainder of the 14 s trial, participants viewed a fixation cross. A total of 70 negative (mean normed valence: 2.60; range: 1.3–3.9), 70 neutral (mean normed valence: 5.05; range: 4.3–5.8), and 70 positive (mean normed valence: 7.30; range: 6.0–8.3) pictures were randomly presented to each participant, for a total of 210 trials.
Incidental Recognition Memory Task
One week following scanning, participants completed an incidental recognition memory task. On each trial, participants first saw a fixation cross for 1000 ms, followed by an IAPS picture probe. The 210 target IAPS pictures they had seen the previous week, as well as 210 foil IAPS pictures, matched for normed intensity and valence, were used as stimuli. The stimulus set designated as “target” or “foil” varied randomly across participants. Participants were instructed to press “1” if they did not remember having seen the picture, “2” if the picture seemed merely familiar, and “3” if participants remembered having seen the picture. Behavioral responses for remembered items were divided into “Hits” (participants had seen the picture during scanning and indicated this during testing) and “False Alarms” (participants had not seen the picture during scanning, but indicated that they had during testing). Hit and False Alarm rates were calculated for each subject by dividing the number of hits and false alarms, respectively, by the total number of “picture seen” responses for each valence category. These rates were then used to compute memory sensitivity indices (d′).
FMRI Data Acquisition
Blood-oxygen level-dependent (BOLD) data were collected on a 1.5T General Electric Signa MR scanner (Milwaukee, Wisconsin), using the following acquisition parameters: 24 slices, spiral-in/out pulse sequence [15], repetition time (TR) = 83 msec/slice, echo time (TE) = 40 msec, flip angle = 70°, field of view (FOV) = 24 cm, acquisition time = 2000 msec/frame. Slices had 3.75 mm2 in-plane and 4 mm through-plane resolution; between-slice distance was 1 mm. Structural scans were collected for co-registration with functional scans using the following parameters: 115 slices, 1 mm2 in-plane and 1.5 mm through-plane resolution, flip angle = 15°, and FOV = 22 cm.
FMRI Data Analysis
Data were preprocessed and analyzed using AFNI (National Institute of Health: http://afni.nimh.nih.gov). BOLD data were slice-time corrected, motion corrected via a Fourier interpolation algorithm, as well as an additional despiking algorithm, spatially smoothed with a Gaussian kernel (full width at half maximum [FWHM] = 4 mm), high-pass filtered with frequency criterion of 1 cycle/min, converted to percent signal change, and, lastly, warped to a common template space [16].
Activity for subsequently remembered negative relative to subsequently remembered neutral were then estimated for each participant. For negative and neutral IAPS, delta functions were computed according to the rule that a picture-viewing event that generated a rating of “3” (picture was seen) during the recognition memory task received a value of 1, and a picture-viewing event that generated a rating of “1” (picture was not seen) during recognition memory testing was given a value of −1. Resulting delta functions were then convolved with a gamma function to render memory-relevant covariates for fitting with voxel-wise BOLD time-courses. Finally, a least-squares data-fitting procedure (AFNI’s 3dDeconvolve) was conducted on the memory covariates, while accounting for nuisance covariates.
To elucidate areas of activation that are predictive of longitudinal symptom change, contrast coefficients of activation differences between subsequently remembered negative versus subsequently remembered neutral pictures were regressed, voxel-wise, against BDI change scores (BDIt2-BDIt1), controlling for initial symptom severity (BDIt1). Correction for multiple comparisons was conducted using a permutation procedure implemented via AFNI’s 3dClustSim, which performs Monte Carlo simulations to estimate the probability of a random field of noise resulting in clusters of a given size. This method compares experimental BOLD data to a simulated noise distribution (null-distribution) in a bootstrapping procedure to assess the likelihood that a cluster of a given size was produced by noise alone, and takes into account the entire voxel-space and smoothing applied to the data. Given our a priori hypotheses, correction for multiple comparisons was conducted across voxels contained within the left and right amygdala (separately) defined using cytoarchitectonic probability maps [17]. The voxel-wise statistical threshold for these analyses was set at α = 0.05, and correction for multiple comparisons (α = 0.05, family-wise error corrected [FWE]) required a cluster threshold k = 5 voxels (135 mm3) and k = 6 voxels (162 mm3) for left and right amygdala, respectively. To identify other possible predictors of symptom change, whole brain analyses were conducted using voxel-wise statistical threshold of α = 0.05 and a cluster correction (k = 71 voxels, 1917 mm3) to account for multiple comparisons. This whole brain analysis resulted in a conservative FWE = p < 0.005, thus substantially reducing the possibility of false positives.
Results
Participants
Nine depressed individuals (6 women, 39.8 ± 8.6 years) participated in this follow-up study. At T1, six were taking one or more antidepressant medications; all participants had maintained a steady antidepressant dosage for at least one month prior to initial assessment, and all reported no history of social phobia, panic disorder, mania, post-traumatic stress disorder, brain injury, lifetime history of primary psychotic ideation, or recent (<6 months) substance abuse. With respect to treatment change from T1 to T2, one participant discontinued pharmacotherapy, one discontinued psychotherapy, and another began psychotherapy. The mean change in BDI scores from T1 to T2 for the full sample was significantly greater than zero [t(8) = 6.70; p < 0.01] (Table 1).
Table 1.
Participant characteristics at Time 1 (T1) and Time 2 (T2)
| Participant | T1 BDI | T2 BDI | BDI Change |
|---|---|---|---|
| MDD1 | 15 | 25 | 10 |
| MDD2 | 24 | 40 | 16 |
| MDD3 | 45 | 38 | −7 |
| MDD4 | 25 | 15 | −10 |
| MDD5 | 15 | 10 | −5 |
| MDD6 | 41 | 45 | 4 |
| MDD7 | 33 | 39 | 6 |
| MDD8 | 39 | 30 | −9 |
| MDD9 | 27 | 13 | −14 |
Behavioral Results
Linear regression analyses, conducted to evaluate the relation between memory sensitivity for neutral and negative stimuli and changes in BDI scores, indicated that the number of successfully recalled negative or neutral images did not predict BDI scores at T2 controlling for BDI scores at T1 (ts < 1.2, ps > 0.26). Further, change in BDI scores after controlling for baseline depression was not predicted by within-subject differences between subsequently remembered negative versus subsequently remembered neutral pictures [t(8) = 0.55, p = 0.60].
Neuroimaging Results
Multiple regression analyses did not yield significant associations between amygdala response to negative versus neutral stimuli at T1 and change in depressive symptoms from T1 to T2. Whole brain analyses, however, yielded significant positive associations between change in depressive symptoms and neural activation in both the posterior cingulate cortex (PCC; Talairach x/y/z coordinates: −2, −49, 24; k = 159 voxels; p < 0.00001) and the medial prefrontal cortex (mPFC; Talairach x/y/z coordinates: 2, 47, 15; k = 77 voxels; p < 0.005; Figure 1). More specifically, the PCC cluster was centered anterior to the subparietal sulcus, and the mPFC cluster was centered on the right medial frontal gyrus, rostral to the anterior end of the cingulate sulcus. Graphical representation of PCC and mPFC activation by BDI change scores indicated that outliers did not drive the effects. Moreover, lowering the threshold to p < 0.1 in our voxel-wise statistical maps yielded no additional clusters, further reducing the possibility that the observed effects in the PCC and mPFC were the result of Type I errors.
Figure 1.
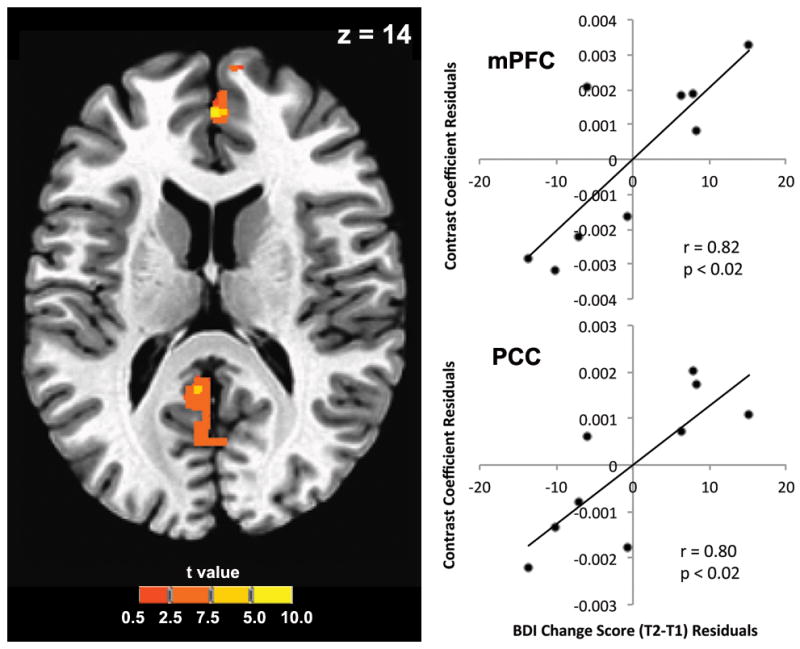
Axial slice showing the posterior cingulate (PCC, k = 159 voxels) and medial prefrontal cortex (mPFC, k = 77 voxels) regions sharing a significant association with symptom change in whole brain multiple regression analyses controlling for initial depression severity. Colorbar indicates t statistic. Brain image shown in neurological convention (right = right).
Finally, exploratory analyses were conducted to elucidate whether discontinuation or changes in psycho- or pharmacotherapies in 3 of the 9 participants in our sample influenced our findings. Partial correlations between mean signal change, extracted separately for each participant from the PCC and mPFC clusters identified in our primary analysis, and BDI score change controlling for baseline depression at T1 showed that the associations between activation in the PCC and mPFC and symptom change remained significant (PCC: r = 0.77, p < 0.05; mPFC: r = 0.78, p < 0.05). Similarly, removing the two participants who had experienced a change in psychotherapy between T1 and T2 showed that the associations between activation in the PCC and mPFC and symptom change remained significant (PCC: r = 0.92, p < 0.01; mPFC: r = 0.86, p < 0.02).
Discussion
Depressed individuals are characterized by better memory for negative material than are nondepressed individuals [1–3]. In the current study, we examined whether heightened activation underlying this bias predicted symptom change 18 months later. While no significant associations were observed between activation in the amygdala and severity of depression at follow-up, whole brain analyses identified the PCC and mPFC as potential biomarkers of naturalistic clinical outcome. That is, greater activation in these regions during the encoding of negative material at T1 was significantly associated with worsening of depressive symptoms (i.e., an increase in BDI scores) at T2. Importantly, this relation between baseline measures and change in depressive symptoms at follow-up was not found for the number of negative items recalled after scanning, indicating that BOLD signal during encoding is a better predictor of long-term symptomatic outcome than is memory sensitivity for negative material. Furthermore, although the size of the sample in the current study is small, it is noteworthy that the effects that were localized the PCC and mPFC were highly significant (p < 0.00001 and p < 0.005, respectively). The robustness of these effects supports the direct involvement of cortical midline activation in the future course of depression.
Our findings add to the existing literature implicating dysfunction of cortical midline regions in MDD [18]. Although it is not clear from the current data exactly how elevated activity in these regions may contribute to poorer recovery in depression, it is noteworthy that both structures represent critical components of the brain’s default mode network [DMN; 19], a system that subserves self-reflection and the appraisal of stimuli as self relevant. Typically, activation of the DMN is suppressed during the performance of a cognitive task [19]. Interestingly, however, neuroimaging studies involving the processing of negatively valenced emotional information find that depressed participants suppress this activation to a far lesser extent than do never-depressed individuals [e.g., 20]. Depression-associated anomalies of the DMN, therefore, may directly contribute to an increase in the self-referential processing of negative material that has been documented in this disorder [21]. Indeed, our findings, combined with behavioral evidence that depressed individuals endorse more negative words as self-relevant than do never-depressed controls [21], and selectively recall more self-relevant negative words than do never-depressed controls [3], provide support for a model whereby negative material is preferentially processed in depression as self relevant and encoded into long-term memory by virtue of an overactive DMN network.
Our findings have important clinical implications. For example, although negative biases in memory have not generally been found to predict depressive course [e.g., 22], therapeutic interventions designed to target DMN-related processes involving self-focus, such as Mindfulness-Based Cognitive Therapy (MBCT), are effective in preventing depressive relapse [e.g., 23]. One interesting future research direction, therefore, could involve examining whether MBCT also alters activation in the PCC and mPFC in depressed individuals.
In a previous study, Canli and colleagues [24] found that greater amygdala activation during the passive viewing of emotional faces (positive or negatve) at baseline predicted symptom improvement in depressed individuals an average of 8 months later. Although both our study and Canli et al.’s investigation found activation to be a better predictor of symptom change than behavioral responses, differences in study design likely accounts for variability in findings. In particular, in creating baseline neural predictors of symptom change, Canli did not sort trials on the basis of whether the emotional images were successfully encoded into long-term memory. This is an important distinction, given that neural reactivity in the amygdala is related to emotional reactivity more generally, which itself has been found to predict greater symptomatic improvement [25]. As we noted above, while the amygdala is involved in memory formation, our identification of the PCC and mPFC as predictors of future symptom change in depression may be attributable to a dependency of memory consolidation on self-referential DMN-based processing.
We should note two limitations of our study. First, the number of subjects assessed at follow-up was relatively small. Although we cannot exclude the possibility of Type II errors, the high significance levels that we obtained in our primary analyses (PCC, p<0.00001; mPFC, p<0.005), in conjunction with exploratory analyses showing (1) that there were no outliers were driving our effects and (2) that no other brain areas shared an association with symptom change in more liberally thresholded maps, suggest that our findings are unlikely to be due to Type I errors. Second, although three of our nine participants reported a change in therapy from T1 to T2, removing these participants did not change our primary findings.
In conclusion, we found that greater activity in the PCC and mPFC of depressed participants as they encoded negative material predicted worsening of symptoms at an 18-month follow-up assessment. These findings suggest that depressed individuals who exhibit elevated PCC and mPFC activation at baseline as they encode mood congruent material require different treatment than do depressed persons who show less robust activation in these regions. Future studies that replicate these findings in larger samples, using procedures that distinguish specific contributions from the DMN and self-relevant processing will have important implications for our understanding of the course of MDD.
Acknowledgments
Funding: This work was supported by grants from the National Institute of Mental Health (MH090617 to LCFR and MH74849 and MH59259 to IHG) and from the Hope for Depression Research Foundation to IHG and LCFR. The funding sources had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
The authors thank Charishma Chotalia for her assistance in data collection.
Footnotes
Conflicts of interest: No author reports any biomedical financial interest or potential conflict of interest.
References
- 1.Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behavior Research and Therapy. 1995;34:865–879. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- 2.Ridout N, Astell AJ, Reid IC, Glen T, O’Carroll RE. Memory bias for emotional facial expressions in major depression. Cognition and Emotion. 2003;17:101–122. doi: 10.1080/02699930302272. [DOI] [PubMed] [Google Scholar]
- 3.Watkins PC, Mathews A, Williamson DA, Fuller RD. Mood-congruent memory in depression: emotional priming or elaboration? J Abnorm Psychol. 1992;101:581–586. doi: 10.1037//0021-843x.101.3.581. [DOI] [PubMed] [Google Scholar]
- 4.Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 5.Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: Evidence from HM and WR. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major sepression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rude SS, Valdez CR, Odom S, Ebrahimi A. Negative cognitive biases predict subsequent depression. Cognitive Therapy and Research. 2003;27:415–429. [Google Scholar]
- 9.Rude SS, Wenzlaff RM, Gibbs B, Vane J, Whitney T. Negative processing biases predict subsequent depressive symptoms. Cognition and Emotion. 2010;16:423–440. [Google Scholar]
- 10.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 11.Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: A meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. doi: 10.1016/j.jad.2011.08.001. in press. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders—Clinician Version (SCID-CV) American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- 13.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 14.Lang PJ, Greenwald MK. International Affective Picture System Standardization Procedure and Results for Affective Judgments: Technical Reports 1A-1C. Center for Research in Psychophysiology, University of Florida; Gainesville, Florida: 1993. [Google Scholar]
- 15.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 16.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: A 3-Dimensional Proportional System, an Approach to Cerebral Imaging. Stuttgart, New York: G. Thieme Medical Publishers; 1988. [Google Scholar]
- 17.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136:e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foland-Ross LC, Hamilton JP, Joormann J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychological Science. 2013;24:334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kircanski K, Mazur H, Gotlib IH. Behavioral activation system moderates self-referent processing following recovery from depression. Psychol Med. doi: 10.1017/S0033291712002851. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SL, Joormann J, Gotlib IH. Does processing of emotional stimuli predict symptomatic improvement and diagnostic recovery from major depression? Emotion. 2007;7:201–206. doi: 10.1037/1528-3542.7.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 24.Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;22:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 25.Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]


