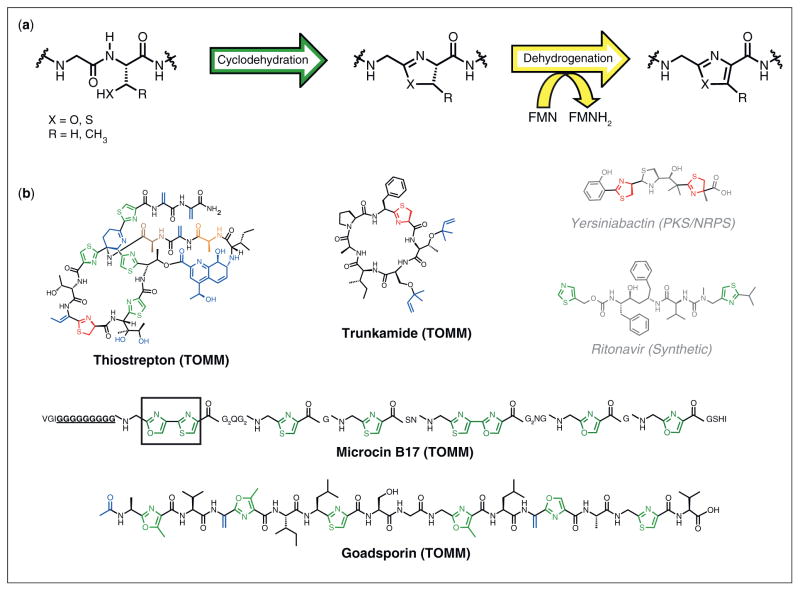
Figure 1.
The biosynthesis and structure of thiazole/oxazole containing compounds. (a) Thiazoles and oxazoles are installed by peptide backbone cyclodehydration of a cysteine, serine, or threonine residue, resulting in a thiazoline or (methyl)oxazoline ring. A FMN-dependent oxidation then yields the thiazole or (methyl)oxazole ring. (b) Representative examples of biologically active thiazole/thiazoline/oxazole-containing compounds. Those that are TOMMs have a black carbon skeleton, while those that are non-TOMMs have a grey carbon skeleton. The latter are either products of a nonribosomal peptide synthetase or are of artificial synthetic origin. Azoles and azolines are colored in green and red, respectively, while other posttranslational modifications are blue. As specifically mentioned in the main text: Microcin B17, the 10-glycine spacer is underlined and bolded, while the first bisheterocycle site is boxed; Thiostrepton, alanines 2 and 4 are highlighted in orange and brown, respectively.