Table 1.
Catalyst and Alcohol Screening
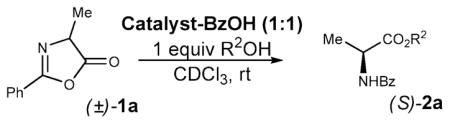
| |||||
|---|---|---|---|---|---|
| entry | catalyst (mol %) | time, d | R2 | % convna | % ee |
| 1 | 11 (5) | 1 | Me | 54 | <3 |
| 2 | 11 (5) | 1 | PhCH2 | 47 | −25 |
| 3 | 11 (5) | 1 | Ph2CH | 5 | ND |
| 4 | 11 (10) | 1 | 1-Np2CH | <5 | ND |
| 5 | 10 (5) | 2 | Me | 91b | 34b |
| 6 | 10 (5) | 2 | PhCH2 | 94b | 48b |
| 7 | 10 (5) | 2 | 1-NpCH2 | 97b | 51b |
| 8 | 10 (5) | 2 | 2-NpCH2 | 96b | 47b |
| 9 | 10 (5) | 2 | Me2CH | <5 | ND |
| 10 | 10 (5) | 2 | Ph2CH | 91b | 75b |
| 11 | 10 (5) | 2 | 1-Np2CH | 96b | 85b |
| 12 | 10 (10) | 0.4 | 1-Np2CH | 92b | 80b |
| 13 | 8 (10) | 2 | 1-Np2CH | 47 | 59 |
| 14 | 9 (10) | 2 | 1-Np2CH | 47 | −52 |
Conversion was determined by 1H NMR, unless indicated otherwise.
Reported % isolated yields and % ee’s are averages of two runs.
