Abstract
Purpose
Few data are available regarding adjuvant trastuzumab use in older women with early-stage breast cancer. We examined rates and predictors of adjuvant trastuzumab completion and cardiac events in this population.
Patients and Methods
We used Surveillance, Epidemiology, and End Results (SEER)-Medicare data to identify patients age ≥ 66 years with stage I to III breast cancer diagnosed between 2005 and 2009 who received trastuzumab. Completion of trastuzumab was defined as receipt of more than 270 days of therapy. We used multivariable logistic regression to examine patient, clinical, and geographic characteristics associated with trastuzumab completion. We also examined rates of hospital admissions for cardiac events.
Results
Among 2,028 women, most (71.2%) were younger than age 76 years and had a comorbidity score of 0 (66.8%); 85.2% received trastuzumab with chemotherapy. Overall, 1,656 women (81.7%) completed trastuzumab. Older patients and those with more comorbidity had lower odds of treatment completion (odds ratio [OR], 0.40 [95% CI, 0.30 to 0.55] for age ≥ 80 years v age 66 to 70 years; OR, 0.65 [95% CI, 0.49 to 0.88] for comorbidity score of 2 v 0). During treatment, 73 patients (3.6%) were hospitalized for cardiac events (2.6% of those who completed trastuzumab v 8.1% of those who did not; P < .001).
Conclusion
Most older patients who initiated adjuvant trastuzumab completed therapy. Age and comorbidity were among factors that were associated with treatment completion, and rates of significant cardiac events were higher in those who did not complete therapy. Further exploration of toxicities and optimal treatments for older women with human epidermal growth factor receptor 2–positive breast cancer are warranted.
INTRODUCTION
HER2/neu (human epidermal growth factor receptor 2) protein overexpression or gene amplification occurs in approximately 25% of primary breast carcinomas and is associated with poor prognosis in the absence of adjuvant treatment.1,2 In 2005, administration of adjuvant trastuzumab became the standard of care for women with HER2/neu-positive breast cancer after results from large randomized trials were presented, demonstrating an approximately 50% reduction in the risk of relapse when trastuzumab was added to standard chemotherapy,3–7 a benefit apparent regardless of patient age.7
Trastuzumab administration is a costly and time-intensive treatment. Although it is possible that patients may benefit from shorter durations of trastuzumab,8 several studies have recently confirmed 1 year of therapy as the standard of care.7,9 Although trastuzumab is generally well tolerated, a risk for cardiotoxicity has been described, especially among patients with pre-existing risk factors, such as older age or low ventricular ejection fraction.10
Currently, data are limited regarding the use and toxicity of trastuzumab outside clinical trials. Available results largely summarize the experiences of younger women, given that they represent the majority of patients enrolled onto breast cancer protocols. A study from the National Comprehensive Cancer Network (NCCN) suggested that adjuvant trastuzumab use may be lower for older women and those with higher comorbidity, but the analysis included relatively few older women.11 In a recent report using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, the adjusted 3-year cumulative incidence of heart failure among older patients with early-stage breast cancer receiving trastuzumab was 32.1 to 41.9 per 100 patients (v 18.1 per 100 patients not treated with trastuzumab).12 Despite these data, we have little understanding of the treatment patterns and feasibility of trastuzumab in our oldest patients. In this study, we examined completion rates of adjuvant trastuzumab and factors associated with treatment completion in a large, population-based cohort of older women. We also characterized the rates of hospitalizations as a result of cardiac events.
PATIENTS AND METHODS
Data Source and Study Population
We used SEER registry data linked with Medicare claims. SEER-Medicare data combine patient and cancer registry data from areas covering 28% of the US population with Medicare administrative data for individuals enrolled in fee-for-service Medicare.13,14 Information on patient demographics, tumor characteristics, treatment use, and mortality for all incident cancers was collected from medical records. The linkage includes matched Medicare enrollment files for 93% of persons age 65 years or older in the SEER registry.14 For this study, we used the Medicare Provider Analysis and Review (MEDPAR) files (inpatient services), the Hospital Outpatient Standard Analytic File (outpatient services), and the 100% Physician/Supplier file (physicians' services and other medical services).
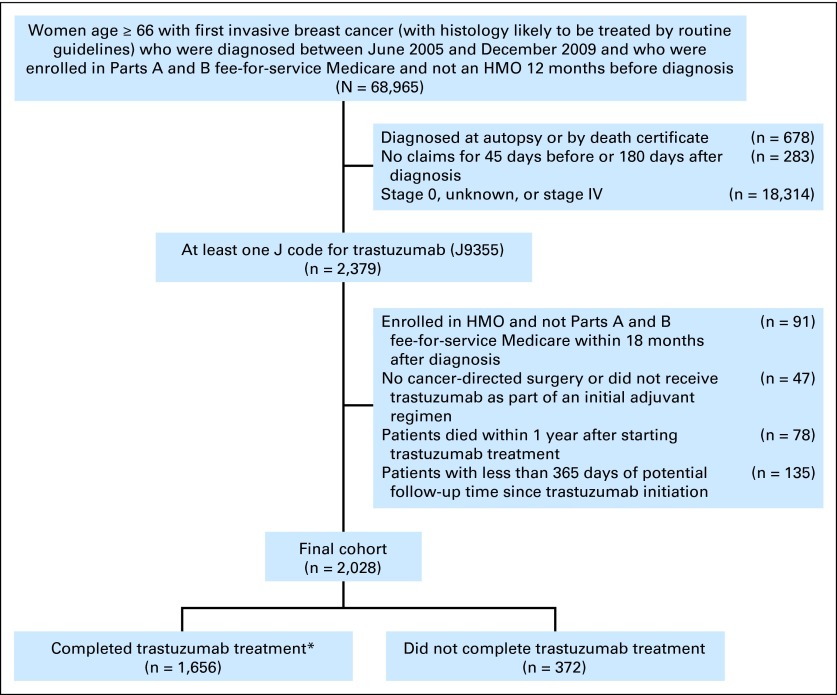
We identified women age ≥ 66 years with a first invasive breast cancer diagnosed from June 2005 to December 2009 who were enrolled in Parts A and B fee-for-service Medicare during the 12 months before diagnosis (N = 68,965). We excluded women diagnosed at autopsy, those with no claims around the time of diagnosis, and those with stage 0, unknown, or stage IV disease (Fig 1). We then restricted the cohort to patients with at least one J code for trastuzumab (J9355; n = 2,379). Finally, we excluded those not enrolled in Parts A and B of fee-for-service Medicare after diagnosis (n = 91), those who did not undergo cancer-directed surgery or did not receive trastuzumab as an initial adjuvant regimen (n = 47), those who died within 1 year after starting trastuzumab (n = 78), and those with less than 365 days of potential follow-up time since trastuzumab initiation (n = 135). In total, 2,028 patients were included in the analytic cohort (Fig 1).
Fig 1.
Flow diagram of patient population. (*) Completion of trastuzumab was defined as more than 270 days of therapy. HMO, health maintenance organization.
Analytic Variables
The primary outcome of interest was completion of adjuvant trastuzumab, defined as receipt of more than 270 days of treatment (women who received ≥ 75% of the recommended 1 year of therapy).11 We calculated the number of days from the first trastuzumab claim (J9355) until the last trastuzumab claim during the first 540 days after the initial trastuzumab claim. This period was selected to include the entire adjuvant course and minimize inclusion of trastuzumab regimens following relapse.
Independent variables included age at diagnosis (66 to 70, 71 to 75, 76 to 80, ≥ 80 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/other/unknown), comorbidity (Charlson index, 0, 1, ≥ 2),15,16 trastuzumab initiation year, median household income, and percent with high school diplomas (based on US Census data, in quartiles; the 1% of patients with missing ZIP Codes were assigned the median values and categorized in quartile 2), marital status (single, separated/divorced/widowed), SEER region (combining registries with similar completion rates given the small sample sizes in many registries: California/Hawaii, Detroit, New Mexico, Atlanta/Rural Georgia, Kentucky/Connecticut/Iowa/Seattle/Utah, Louisiana, New Jersey), location of residence (major metropolitan, metropolitan/urban, less urban/rural), tumor size (0 to 2, 2.1 to 3, > 3 cm, unknown), number of positive nodes (0, 1 to 3, 4 to 9, ≥ 10, unknown), tumor grade (low/intermediate, high), hormone receptor status (positive if estrogen receptor–positive or progesterone receptor–positive; negative if estrogen receptor–negative and progesterone receptor–negative; the 5% of patients with unknown hormone receptor status were considered hormone receptor–positive, consistent with receptor status for most patients with breast cancer),14 type of surgery (lumpectomy, mastectomy), and type of chemotherapy (anthracycline-taxane based, anthracycline-based, taxane-based multidrug regimen, single taxane, other chemotherapy, no chemotherapy; Appendix, online only).
Cardiac Hospitalizations
We assessed hospitalizations for cardiac events during two periods: (1) the time from the first until the last dose of trastuzumab + 30 days and (2) 1 year after the last dose of trastuzumab. Our primary outcome of interest was hospitalization with a primary diagnosis of congestive heart failure (International Classification of Diseases, 9th revision [ICD-9] for heart failure or cardiomyopathy).12 To be more inclusive, we also examined admissions for conduction disorders and dysrhythmia, myocardial infarction and angina, and ill-defined cardiac conditions (Appendix).
Statistical Analysis
We compared rates of trastuzumab completion by prespecified independent variables using χ2 tests. We then performed a logistic regression model for treatment completion with generalized estimating equations to account for clustering within the SEER registry, controlling for all of the independent variables described earlier.
We performed several sensitivity analyses. First, we repeated the model after redefining completion of trastuzumab as receipt of more than 350 days of treatment. Second, we repeated the model after redefining completion of trastuzumab as receipt of more than 270 days of treatment with at least 13 trastuzumab claims (≥ 75% of expected claims for a once-every-3-weeks schedule). Third, we excluded women with fewer than 540 days of claims after initiation of trastuzumab (n = 290) to account for women whose treatment may have extended beyond 1 year. Fourth, because of the uncertainty of reasons for treatment breaks, we repeated analyses after excluding 165 women who had a ≥ 60-day break in treatment without evidence of receipt of anthracycline or surgery during that break. Finally, we also performed a model that included patients who died within 1 year after starting trastuzumab.
To examine toxicity patterns for women receiving trastuzumab, we used χ2 tests to compare the rates of cardiac hospitalizations (from the first trastuzumab claim through 30 days after the last trastuzumab claim and during 1 year after the last trastuzumab claim) in women who completed treatment with those who did not complete treatment. We also examined the rates of cardiac hospitalizations by prior cardiac history, defined as a history of cardiac admission or as a diagnosis of heart failure at time of diagnosis as coded by the Charlson comorbidity index.15,16 To examine how cardiac admissions that occurred during treatment may have had an impact on the occurrence of future cardiac events, we also examined the rates of cardiac hospitalizations during the year after the final trastuzumab claim for women who were admitted for a cardiac event during the course of treatment (v those who were not admitted during treatment). We then performed a series of logistic regression models examining the associations of treatment completion and prior cardiac hospitalization with cardiac events during treatment through 30 days after the last claim and during the year after the last trastuzumab claim. First, we examined the association of all causes of cardiac admissions and then examined heart failure or cardiomyopathy admissions specifically. We adjusted for relevant clinical and socioeconomic variables. We then performed sensitivity analyses that included patients who died within 1 year after starting trastuzumab, because excluding these women could lead to underestimation of relevant admissions.
All statistical analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, NC). The study was considered exempt from review by the institutional review board at Dana-Farber Cancer Institute.
RESULTS
Cohort Characteristics
Patient and tumor characteristics are presented in Table 1. Most of the 2,028 women (71.2%) were younger than age 76 years, and 66.8% had a comorbidity score of 0; 11.1% had a comorbidity score ≥ 2. Most patients were non-Hispanic white (82.3%). With regard to the chemotherapy component of treatment, 24.7% received anthracycline-taxane–based therapy, 41.4% taxane-based multidrug regimen therapy, 9.7% single-agent taxane, 6.1% anthracycline-based therapy, 3.4% another chemotherapy agent, and 14.8% trastuzumab without chemotherapy.
Table 1.
Patient Demographic and Clinical Characteristics Stratified by Completion of Trastuzumab
| Characteristic | Full Cohort |
% Completing Trastuzumab | P* | |
|---|---|---|---|---|
| No. | % | |||
| Overall | 2,028 | 100 | 81.7 | |
| Age at diagnosis, years | < .001 | |||
| 66-70 | 833 | 41.1 | 86.1 | |
| 71-75 | 610 | 30.1 | 82.8 | |
| 76-80 | 362 | 17.9 | 77.4 | |
| ≥ 80 | 223 | 11.0 | 69.1 | |
| Race/ethnicity | .01 | |||
| Non-Hispanic white | 1,668 | 82.3 | 82.6 | |
| Non-Hispanic black | 143 | 7.1 | 72.7 | |
| Hispanic/other/unknown | 217 | 10.7 | 80.7 | |
| SEER region† | .86 | |||
| California/Hawaii | 684 | 33.7 | 81.0 | |
| Detroit | 87 | 4.3 | 81.6 | |
| New Mexico | 50 | 2.5 | 88.0 | |
| Atlanta/Rural Georgia | 279 | 13.8 | 82.1 | |
| Kentucky/Connecticut/Iowa/Seattle/Utah | 500 | 24.7 | 82.8 | |
| Louisiana | 132 | 6.5 | 81.1 | |
| New Jersey | 296 | 14.6 | 80.1 | |
| Location of residence | .35 | |||
| Major metropolitan | 1,079 | 53.2 | 81.2 | |
| Metropolitan/urban | 756 | 37.3 | 81.4 | |
| Less urban/rural | 193 | 9.5 | 85.5 | |
| Socioeconomic status, median income (quartile) | .33 | |||
| 1 (lowest) | 486 | 24.0 | 82.1 | |
| 2 | 522 | 25.7 | 79.1 | |
| 3 | 508 | 25.1 | 82.1 | |
| 4 (highest) | 512 | 25.3 | 83.4 | |
| High school graduation rates (quartile) | .98 | |||
| 1 (lowest) | 503 | 24.8 | 81.9 | |
| 2 | 508 | 25.1 | 81.5 | |
| 3 | 508 | 25.1 | 82.1 | |
| 4 (highest) | 509 | 25.1 | 81.1 | |
| Marital status | .01 | |||
| Single‡ | 996 | 49.1 | 79.4 | |
| Married | 1,032 | 50.9 | 83.8 | |
| Charlson comorbidity score | .03 | |||
| 0 | 1,354 | 66.8 | 83.0 | |
| 1 | 449 | 22.1 | 80.4 | |
| ≥ 2 | 225 | 11.1 | 76.0 | |
| Year of initiation of trastuzumab | .044 | |||
| 2009 | 550 | 27.1 | 82.7 | |
| 2008 | 481 | 23.7 | 85.7 | |
| 2007 | 510 | 25.2 | 79.2 | |
| 2006 | 425 | 21.0 | 79.3 | |
| 2005 | 62 | 3.1 | 77.4 | |
| Tumor size, cm | .71 | |||
| 0-2 | 936 | 46.2 | 82.6 | |
| 2.1-3 | 526 | 25.9 | 81.6 | |
| > 3 | 518 | 25.5 | 80.1 | |
| Unknown | 48 | 2.4 | 81.3 | |
| No. of positive nodes | .03 | |||
| 0 | 975 | 48.1 | 84.4 | |
| 1-3 | 544 | 26.8 | 79.4 | |
| 4-9 | 260 | 12.8 | 80.4 | |
| ≥ 10 | 136 | 6.7 | 77.2 | |
| Unknown | 113 | 5.6 | 77.0 | |
| Stage | .08 | |||
| I | 604 | 29.8 | 84.6 | |
| II | 898 | 44.3 | 80.6 | |
| III | 526 | 25.9 | 80.0 | |
| Tumor grade | .87 | |||
| High | 1,294 | 63.9 | 81.5 | |
| Low/intermediate | 734 | 36.1 | 81.8 | |
| Hormone receptor status§ | .83 | |||
| Positive | 1,182 | 58.3 | 81.8 | |
| Negative | 846 | 41.7 | 81.4 | |
| Surgery | .01 | |||
| Lumpectomy | 853 | 42.1 | 84.2 | |
| Mastectomy | 1,175 | 57.9 | 79.8 | |
| Chemotherapy | < .001 | |||
| Anthracycline/taxane based | 500 | 24.7 | 78.6 | |
| Anthracycline based | 123 | 6.1 | 84.6 | |
| Taxane-based multidrug regimen | 839 | 41.4 | 87.3 | |
| Taxane only | 197 | 9.7 | 74.1 | |
| Other chemotherapy | 69 | 3.4 | 73.9 | |
| No chemotherapy | 300 | 14.8 | 76.7 | |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
P calculated by χ2 testing for characteristics associated with completion rate.
Regions were collapsed because of small sample sizes.
Single, separated, widowed, and divorced categories were collapsed.
Defined as positive if patient was estrogen receptor–positive or progesterone receptor–positive. Defined as negative if patient was estrogen receptor–negative or progesterone receptor–negative, and neither were positive. Patients with unknown hormone receptor status were considered hormone receptor positive.
Completion of Adjuvant Trastuzumab
Among 2,028 women, 1,656 women (81.7%) completed ≥ 270 days of treatment, with a median duration of adjuvant trastuzumab treatment of 357 days (range, 1 to 721 days). Patients age ≥ 80 years were less likely than others to complete adjuvant trastuzumab, as were patients with comorbidities (Fig 2). Patients who received a taxane-based multidrug regimen had the highest completion rates of adjuvant trastuzumab (87.3%; P < .001; Table 1).
Fig 2.
Unadjusted adjuvant trastuzumab completion rates (95% CIs) by (A) age and (B) comorbidity; (C) trastuzumab completion rates by age and comorbidity (comorbidity includes patients with 1 and 2+ comorbidities). Y-axes indicate age groups, left, and hazard ratios with CIs, right. Overall P values determined by χ2 testing.
In the multivariable analysis (Table 2), older women, women of Hispanic/other/unknown ethnicity versus white, and those with a comorbidity score of 1 or 2+ versus 0 had a lower likelihood of trastuzumab completion. Patients who received taxane-based multidrug therapy or anthracycline-based therapy had higher odds of treatment completion than patients who received anthracycline-taxane regimens. Results of all sensitivity analyses for treatment completion were similar (data not shown).
Table 2.
Multivariable Logistic Regression Analysis for Completion of Trastuzumab Treatment in > 270 Days
| Variable | OR | 95% CI | P* |
|---|---|---|---|
| Age at diagnosis, years | < .001 | ||
| 66-70 | 1.00 | ||
| 71-75 | 0.79 | 0.60 to 1.04 | |
| 76-80 | 0.57 | 0.35 to 0.91 | |
| ≥ 80 | 0.40 | 0.30 to 0.55 | |
| Race/ethnicity | .002 | ||
| Non-Hispanic white | 1.00 | ||
| Non-Hispanic black | 0.83 | 0.64 to 1.08 | |
| Hispanic/other/unknown | 0.56 | 0.40 to 0.78 | |
| SEER region† | < .001 | ||
| California/Hawaii | 1.00 | ||
| Detroit | 1.26 | 1.06 to 1.49 | |
| New Mexico | 1.85 | 1.55 to 2.21 | |
| Atlanta/Rural Georgia | 1.11 | 1.00 to 1.23 | |
| Kentucky/Connecticut/Iowa/Seattle/Utah | 1.13 | 0.90 to 1.43 | |
| Louisiana | 1.29 | 1.14 to 1.47 | |
| New Jersey | 0.99 | 0.93 to 1.06 | |
| Location of residence | .40 | ||
| Major metropolitan | 1.00 | ||
| Metropolitan/urban | 0.91 | 0.70 to 1.20 | |
| Less urban/rural | 1.12 | 0.81 to 1.54 | |
| Socioeconomic status, median income (quartile) | .02 | ||
| 1 (lowest) | 1.00 | ||
| 2 | 0.78 | 0.65 to 0.95 | |
| 3 | 0.94 | 0.68 to 1.30 | |
| 4 (highest) | 1.14 | 0.86 to 1.53 | |
| High school graduation rates (quartile) | .14 | ||
| 1 (lowest) | 1.00 | ||
| 2 | 1.42 | 1.05 to 1.94 | |
| 3 | 1.35 | 1.02 to 1.79 | |
| 4 (highest) | 1.29 | 0.83 to 2.00 | |
| Marital status | .40 | ||
| Single‡ | 1.00 | ||
| Married | 0.90 | 0.71 to 1.14 | |
| Charlson comorbidity score | .02 | ||
| 0 | 1.00 | ||
| 1 | 0.82 | 0.70 to 0.96 | |
| ≥ 2 | 0.65 | 0.49 to 0.88 | |
| Year of initiation of trastuzumab | .006 | ||
| 2009 | 1.00 | ||
| 2008 | 1.26 | 1.00 to 1.58 | |
| 2007 | 0.89 | 0.66 to 1.21 | |
| 2006 | 0.96 | 0.68 to 1.35 | |
| 2005 | 0.87 | 0.44 to 1.69 | |
| Tumor size, cm | .45 | ||
| 0-2 | 1.00 | ||
| 2.1-3 | 1.10 | 0.90 to 1.35 | |
| > 3 | 1.15 | 0.86 to 1.54 | |
| Unknown | 1.49 | 0.82 to 2.69 | |
| No. of positive nodes | .27 | ||
| 0 | 1.00 | ||
| 1-3 | 0.86 | 0.64 to 1.15 | |
| 4-9 | 0.98 | 0.68 to 1.43 | |
| ≥ 100 | 0.84 | 0.52 to 1.35 | |
| Unknown | 0.73 | 0.47 to 1.14 | |
| Tumor grade | .67 | ||
| High | 1.00 | ||
| Low/intermediate | 1.05 | 0.83 to 1.33 | |
| Hormone receptor status§ | .53 | ||
| Positive | 1.00 | ||
| Negative | 0.97 | 0.87 to 1.07 | |
| Surgery | .05 | ||
| Mastectomy | 1.00 | ||
| Lumpectomy | 1.24 | 1.00 to 1.54 | |
| Chemotherapy | < .001 | ||
| Anthacycline-taxane‖ | 1.00 | ||
| Anthracycline based¶ | 1.57 | 1.04 to 2.37 | |
| Taxane-based multidrug regimen# | 1.98 | 1.66 to 2.37 | |
| Taxane only | 1.06 | 0.76 to 1.49 | |
| Other chemotherapy | 0.97 | 0.60 to 1.55 | |
| No chemotherapy | 1.28 | 0.97 to 1.68 |
NOTE. Multivariable logistic regression was used, accounting for clustering at registry level and adjusting for all variables in the table.
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
P calculated by Wald test.
Regions collapsed because of small sample sizes.
Single, separated, widowed, and divorced categories were collapsed.
Defined as positive if estrogen receptor–positive or progesterone receptor–positive. Defined as negative if estrogen receptor–negative or progesterone receptor–negative, and neither were positive. Patients with unknown hormone receptor status were considered hormone receptor positive.
The majority of patients received doxorubicin, cyclophosphamide, and paclitaxel.
The majority of patients received doxorubicin and cyclophosphamide.
The majority of patients received docetaxel and carboplatin.
Cardiac Hospitalizations
During treatment through 30 days after the last trastuzumab claim, 73 patients (3.6%) had 84 hospital admissions for cardiac events (2.6% in those who completed trastuzumab v 8.1% in those who did not; P < .001). Among women who were hospitalized for cardiac events, the median time to admission was shorter in the group that did not complete treatment versus the group that completed treatment (65 days v 217 days; P < .001). Patients who had a history of cardiac disease had a higher likelihood of requiring a cardiac admission during treatment (ie, 9.6% who had a prior cardiac hospitalization were hospitalized v 2.7% of women without prior cardiac hospitalization [P < .001]; 10.3% of those with prior heart failure were hospitalized v 3.4% of those without heart failure [P = .002]). Patients who completed treatment were less likely than those who did not complete treatment to have a cardiac event requiring admission during treatment (odds ratio, 0.32; 95% CI, 0.21 to 0.49; Table 3). Overall, 56.0% of the cardiac admissions were followed by another trastuzumab claim (ie, therapy was not stopped), but only 25% of the cardiac admissions in those who did not complete trastuzumab were followed by a trastuzumab claim.
Table 3.
Risk of Any Cardiac Admission and Specific Types of Admissions During or Following Trastuzumab Therapy
| Variable | Any Cardiac Admission |
Heart Failure/Cardiomyopathy Admission |
Other Cardiac Admission |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % | OR* | 95% CI* | % | OR* | 95% CI* | % | OR* | 95% CI* | |
| Cardiac admission during treatment + 30 days (N = 2,028)† | 3.6 | 1.2 | 2.4 | ||||||
| Completed trastuzumab | |||||||||
| No (n = 372) | 8.1 | 1.00 | 4.0 | 1.00 | 4.0 | 1.00 | |||
| Yes (n = 1,656) | 2.6 | 0.32 | 0.21 to 0.49 | < 1.5‡ | 0.14 | 0.09 to 0.23 | 2.1 | 0.57 | 0.31 to 1.02 |
| Cardiac admission in the year following the last trastuzumab dose (N = 2,028)§ | 4.0 | 1.7 | 2.5 | ||||||
| Completed trastuzumab | |||||||||
| No (n = 372) | 11.6 | 1.00 | 6.7 | 1.00 | 5.4 | 1.00 | |||
| Yes (n = 1,656) | 2.3 | 0.22 | 0.12 to 0.41 | < 1.5‡ | 0.09 | 0.02 to 0.37 | 1.8 | 0.36 | 0.18 to 0.71 |
| Hospitalized during trastuzumab treatment | |||||||||
| Yes (n = 73) | 50.7 | 1.0 | 20.6 | 1.00 | 34.3 | 1.00 | |||
| No (n = 1,955) | 2.3 | 45.9 | 22.7 to 93.2 | 1.0 | 334.9 | 43.5 to 2,575.75 | 1.3 | 94.40 | 36.77 to 242.36 |
Abbreviation: OR, odds ratio.
Adjusting by age, race/ethnicity, comorbidity, trastuzumab initiation year, median household income, percent who graduated from high school, marital status, location of residence, and prior cardiac admission (yes or no). Patients with prior cardiac admissions and higher comorbidity were more likely to have a cardiac admission in both periods.
Calculated from first day of trastuzumab treatment until 30 days after the last trastuzumab claim. During treatment + 30 days after the last trastuzumab claim, 73 patients had 84 hospital admissions for cardiac events. The reasons for admissions (heart failure/cardiomyopathy v other cardiac admission) do not add up to 84 admissions because there were patients who were admitted for the same reason more than one time.
Exact proportion was not provided because of small cell sizes.
Calculated until 365 days after last trastuzumab claim. In the year after the last trastuzumab claim, 81 patients had 101 hospital admissions for cardiac events. The reasons for admissions (heart failure/cardiomyopathy v other cardiac admission) do not add up to 101 admissions because there were patients who were admitted for the same reason more than one time.
During the year after the last adjuvant trastuzumab claim, 81 patients (4.0%) had 101 hospital admissions for cardiac events (2.3% in those who completed trastuzumab v 11.6% in those who did not; P < .001). The rates for heart failure/cardiomyopathy admission were significantly higher in those who did not complete trastuzumab than in those who did (6.7% v < 1.5%; P < .001). The rates of hospital admissions during the year after stopping trastuzumab were higher in women with prior hospitalizations compared with women without (50.7% v 2.3%; P < .001; Table 3), consistent with the known high rates of cardiac readmission among Medicare beneficiaries.17 In the multivariable analysis, these associations persisted (Table 3). Results of all sensitivity analyses were similar (data not shown).
DISCUSSION
In this large cohort of older patients receiving adjuvant trastuzumab, 81.7% completed 1 year of therapy. Despite these high treatment completion rates, we observed lower odds of completion in some subgroups (eg, 69.1% of patients older than age 80 years completed treatment). We also found that 3.6% and 4.0% of women were hospitalized with cardiac diagnoses during trastuzumab treatment and in the year after treatment, respectively.
Few data are available about the use of adjuvant trastuzumab in older women with early-stage breast cancer. In the seminal adjuvant trials of trastuzumab, older patients were underrepresented, and although 9% to 31% of patients discontinued trastuzumab treatment before completion, the proportion of older women not completing treatment is unclear.3–5 Our results are consistent with those among older women treated at NCCN centers, where age and comorbidity also had an impact on trastuzumab use.11 Among 105 women age ≥ 70 years with HER2-positive disease in the NCCN, 54% initiated trastuzumab-based treatment and 73% of these women completed treatment, generally similar to our observed rates of treatment completion. Interestingly, although age and comorbidity were both associated with lower initiation rates of trastuzumab among women with HER2-positive breast cancer within the NCCN, these factors were not significantly associated with trastuzumab completion. However, that analysis included relatively few older women, lacked detailed information on toxicity, and focused only on women treated at academic medical centers.
In our analysis, patients with comorbidity scores of 1 or 2+ were less likely to complete treatment than patients with scores of 0. Other studies have also demonstrated that providers are less likely to recommend adjuvant treatment for patients with increasing age and/or deteriorating health status.18,19 Therefore, it is not surprising (and likely appropriate) that these factors are playing a role in early discontinuation of adjuvant trastuzumab.
In addition to comorbidity and age, we observed differences in trastuzumab completion by regimen received. Women receiving taxane-based regimens and anthracycline-based regimens had higher odds treatment completion compared with anthracycline-taxane-based regimens. It is conceivable that these differences are explained by better overall tolerance to treatment in women not receiving combination regimens. However, we did not have detailed information on toxicity, and in the absence of a randomized comparison, it is equally likely that selection factors played a role in this observation. For example, patients receiving single-taxane trastuzumab therapy may have other health factors not captured in the comorbidity score that predispose to toxicity. In addition, we did not have information on the thresholds for providers and patients to discontinue treatment. In the National Surgical Adjuvant Breast and Bowel Project NSABP-B-31/North Central Cancer Treatment Group NCCTG-N9831 trial, among the 31.4% of patients who discontinued treatment, the reasons for discontinuation included cardiac toxicity (60%), noncardiac toxicity (7%), patient-initiated discontinuation (19%), recurrence (6%), and other reasons (7%).4
A systematic review suggests that the rate of severe heart failure is higher for patients treated with trastuzumab than for patients not treated with trastuzumab (2.5% v 0.4%),7 although the rate of any trastuzumab-related cardiac toxicity in clinical trials has been low (1% to 4%).3,4,8,10,20 Nevertheless, recent population-based studies have raised concerns that the incidence of heart failure may be higher outside clinical trials, particularly for older women.12,21 Our results suggest that cardiac admissions may be an important cause of treatment discontinuation for older women, since those who did not complete trastuzumab had significantly higher rates of cardiac admissions than those who completed treatment (8.1% v 2.6%) and had a lower likelihood of continuing trastuzumab after hospital admission. In addition, we observed dramatically higher rates of cardiac readmission in patients who had a cardiac hospitalization during trastuzumab treatment than in those without cardiac events during treatment, raising concern for ongoing risk in these patients (50.7% v 2.3%).
Our study has several strengths. To the best of our knowledge, this is the largest study to date examining trastuzumab treatment patterns in older women, and we were also able to examine how demographic, clinicopathologic, and treatment factors affect trastuzumab completion and toxicity in a national cohort. However, we acknowledge several limitations. First, HER2 status was not available; therefore, we were unable to assess the rate of trastuzumab initiation for all patients with HER2-positive cancers. Because providers and patients may not initiate trastuzumab-based therapy when comorbidities and competing risks are present, our results are limited to those who initiated treatment. Second, although our definition of completion (75% of treatment), is somewhat arbitrary, our results did not change despite changing the definition for completion in several sensitivity analyses. Third, detailed information on why trastuzumab was stopped or why treatment breaks occurred was not available, nor did our analyses account for factors that could influence treatment, such as social supports or patient and provider preferences. Furthermore, given the limitations of registry and claims data, we were not able to establish whether treatment stopped because of a cardiac event. Fourth, because of the small numbers of women in some subgroups, we may have had insufficient power to detect differences in treatment completion. Fifth, non-SEER geographic areas were not represented in our analysis, and we consolidated some geographic regions because of small sample sizes. Sixth, there is the possibility of misclassification of chemotherapy regimens.22 Finally, cardiac events were defined on the basis of primary ICD-9 codes for hospital admissions only and did not capture asymptomatic decreases in ejection fraction or mild symptoms of cardiac events that did not require hospitalization. However, we were able to capture significant cardiac events, which is still informative.
In conclusion, although trastuzumab completion rates were generally high for most women, sicker and older patients were less likely to complete treatment, and rates of cardiac events were not rare. Our observation that 18% of older patients did not complete trastuzumab treatment is particularly interesting in light of the recent studies suggesting that 1 year of adjuvant trastuzumab treatment is superior to 6 months of treatment.7,9 Although decisions to discontinue therapy may be appropriate in many cases because of issues with treatment tolerance or coexisting medical conditions, a better understanding of treatment toxicity and efficacy in older women is crucial. In parallel maximizing inclusion of older patients into clinical trials, developing more tolerable regimens and expanding research efforts that allow linkage of individualized clinical, functional, and toxicity information to patient biologic samples would be extremely informative. An example of this effort is the upcoming Alliance for Clinical Trials study that will examine trastuzumab emtansine as adjuvant treatment in an older population. This improved knowledge will allow us to incorporate the personalized risks and benefits of treatment into discussions and decisions and will optimize the delivery of guideline-recommended care. With the expected substantial improvement in breast cancer outcomes for women who complete 1 year of adjuvant trastuzumab, appropriate and tailored administration of this agent could have a significant impact on disease recurrence and survival for women with HER2-positive breast cancer.
Acknowledgment
We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Glossary Terms
- HER2/neu (human epidermal growth factor receptor-2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- Surveillance, Epidemiology, and End Results (SEER):
a national cancer registry that collects information from all incident malignancies in multiple geographic areas of the United States.
- trastuzumab:
a humanized anti-ErbB2 monoclonal antibody approved for treating patients whose breast cancers overexpress the ErbB2 protein or demonstrate ErbB2 gene amplification. It is currently being tested in combination with other therapies.
Appendix
Definition of Type of Chemotherapy
The following J codes were flagged: bevacizumab: J9035; capecitabine: J8520, J8521; carboplatin: J9045; cisplatin: J9060, J9062; cyclophosphamide: J8530, J9070, J9080, J9091, J9092, J9093, J9094, J9095, J9096, J9097; docetaxel: J9170, J9171; doxorubicin: J9000, J9001; epirubicin: J9178, J9180; gemcitabine: J9201; ixabepilone: J9207; methotrexate: J8610, J9250, J9260; paclitaxel: J9265, J9264; vinorelbine: J9390; fluorouracil: J9190.
The regimen assigned was the first regimen received by the patient. Sequential regimens allowed a break between different types of chemotherapy of less than 90 days. Several chemotherapy categories were assigned.
The anthracycline-taxane–based regimens included doxorubicin-cyclophosphamide-docetaxel; doxorubicin-cyclophosphamide-paclitaxel; doxorubicin-paclitaxel; docetaxel-doxorubicin-cyclophosphamide; epirubicin-cyclophosphamide-paclitaxel; docetaxel-fluorouracil-epirubicin-cyclophosphamide; fluorouracil-epirubicin-cyclophosphamide-docetaxel; fluorouracil-epirubicin-cyclophosphamide-paclitaxel; and cyclophosphamide-docetaxel-epirubicin.
The anthracycline-based regimens included doxorubicin-cyclophosphamide; epirubicin-cyclophosphamide; cyclophosphamide-epirubicin-fluorouracil; fluorouracil-epirubicin-cyclophosphamide; and doxorubicin.
The taxane-based multidrug regimens included docetaxel-cyclophosphamide; docetaxel- carboplatin-cyclophosphamide; docetaxel-carboplatin-bevacizumab; carboplatin-paclitaxel; capecitabine-docetaxel; docetaxel-bevacizumab; paclitaxel-cyclophosphamide; paclitaxel-bevacizumab; and carboplatin-cyclophosphamide-paclitaxel.
The single-taxane regimens included docetaxel and paclitaxel.
Other chemotherapy regimens included cyclophosphamide-methotrexate-fluorouracil; fluorouracil; carboplatin; carboplatin-vinorelbine; gemcitabine-vinorelbine; gemcitabine; and vinorelbine.
The “no chemotherapy” regimens signified trastuzumab only.
International Classification of Diseases, 9th Revision, Codes Used for Cardiac Admissions
The following codes were used for heart failure or cardiomyopathy: 402.XX, 404.XX, 425.XX, and 429.3; conduction disorders and dysrhythmia: 426.XX and 427.XX; myocardial infarction and angina: 410 to 414.XX; and ill-defined cardiac conditions: 429.8 and 429.9.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Interpretation and reporting of data from the linked SEER-Medicare database are the sole responsibility of the authors. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Public Health; the National Cancer Institute; and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should it be inferred.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Support
Supported by Grant No. NIH P50 CA089393 from the National Cancer Institute Specialized Program of Research Excellence in Breast Cancer (E.P.W.), ACT NOW fund, Susan G. Komen for the Cure (E.P.W. and N.L.K.), Grant No. HMSP-ICS/0004/2011 from the Fundacao para a Ciencia e Tecnologia Career Development Award (I.V.-L.), and a grant from the Dana-Farber Cancer Institute Friends (R.A.F.). Supported by the California Department of Public Health (for collection of the California cancer incidence data) as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under Contract No. N01-PC-35136 (Northern California Cancer Center), Contract No. N01-PC-35139 (University of Southern California), and Contract No. N02-PC-15105 (Public Health Institute); and Agreement No. U55/CCR921930-02 from the Centers for Disease Control and Prevention's National Program of Cancer Registries (Public Health Institute).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Nancy U. Lin, Genentech (U); Eric P. Winer, Genentech (U) Stock Ownership: None Honoraria: None Research Funding: Nancy U. Lin, Genentech, GlaxoSmithKline, Novartis, Array BioPharma; Eric P. Winer, Genentech Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ines Vaz-Luis, Nancy L. Keating, Nancy U. Lin, Rachel A. Freedman
Financial support: Ines Vaz-Luis, Rachel A. Freedman
Administrative support: Ines Vaz-Luis, Rachel A. Freedman
Provision of study materials or patients: Rachel A. Freedman
Collection and assembly of data: Ines Vaz-Luis, Nancy L. Keating, Huichuan Lii, Rachel A. Freedman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Untch M, Gelber RD, Jackisch C, et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol. 2008;19:1090–1096. doi: 10.1093/annonc/mdn005. [DOI] [PubMed] [Google Scholar]
- 7.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 9.Pivot X, Romieu G, Bonnefoi H, et al. PHARE Trial results of subset analysis comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer. Cancer Res. 2012;(suppl 24):72. abstr S5-3. [Google Scholar]
- 10.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–846. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40 doi: 10.1097/00005650-200208001-00009. IV-62-8. [DOI] [PubMed] [Google Scholar]
- 14.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 17.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: The perspective of oncologists and primary care providers. J Clin Oncol. 2008;26:5386–5392. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurria A, Wong FL, Pal S, et al. Perspectives and attitudes on the use of adjuvant chemotherapy and trastuzumab in older adults with HER-2+ breast cancer: A survey of oncologists. Oncologist. 2009;14:883–890. doi: 10.1634/theoncologist.2009-0056. [DOI] [PubMed] [Google Scholar]
- 20.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund JL, Stürmer T, Harlan LC, et al. Identifying specific chemotherapeutic agents in Medicare data: A validation study. Med Care. 2013;51:e27–e34. doi: 10.1097/MLR.0b013e31823ab60f. [DOI] [PMC free article] [PubMed] [Google Scholar]