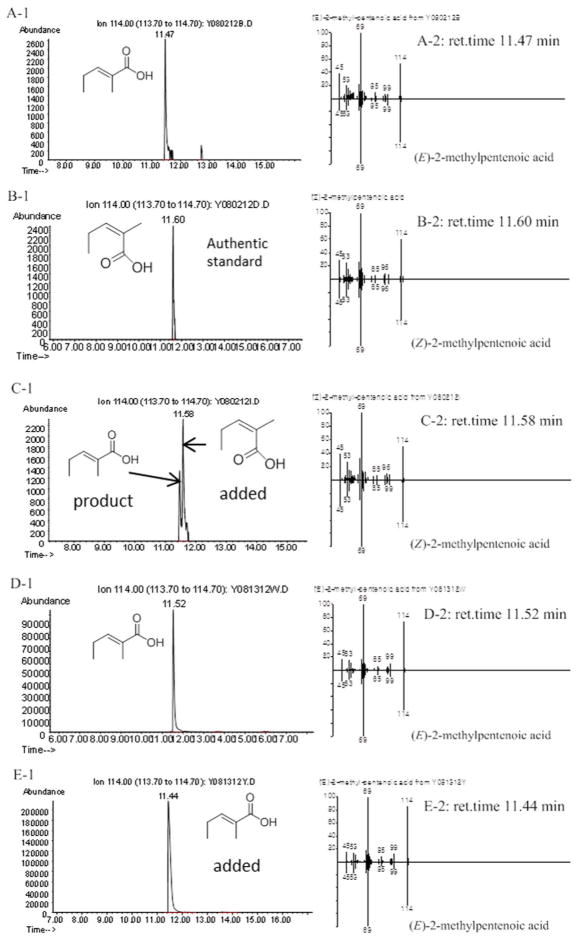
Figure 3.
Chiral GC-MS analysis (Method 1) of the incubation of (2RS)-2-methyl-3-ketopentanoyl-RifACP10-NusA with RifKR7 and RifDH10. A and D: (E)-2-Methyl-2-pentenoic acid (3) from RifKR7-catalyzed reduction of (2RS)-2-methyl-3-ketopentanoyl-RifACP10-NusA followed by dehydration by RifDH10. B: (Z)-2-methylpentenoic acid authentic standard. C: Co-injection of A with B. E: Co-injection of (E)-2-methyl-2-pentenoic acid standard with D. A-1, B-1, C-1, D-1 and E-1: Extracted ion current (XIC) at m/z 114 (base peak). A-2, B-2, C-2, D-2 and E-2: mass spectra of selected peak, upper half, observed spectrum, lower half, inverted mass spectrum of reference standard.