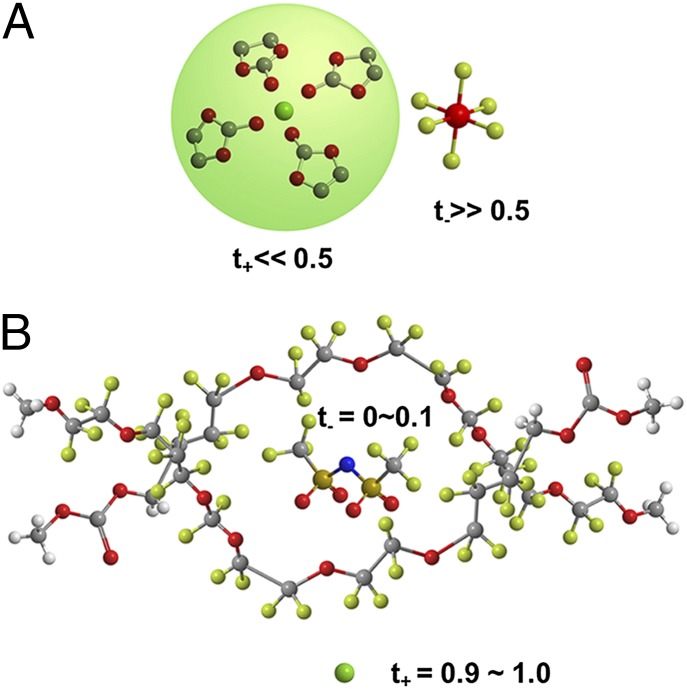
Fig. 2.
(A) In conventional nonaqueous electrolytes, Li+ is tightly solvated and slowed down in its migration by coordinating carbonate molecules, resulting in low t+. (B) In electrolytes based on highly fluorinated solvents, anion TFSI is solvated instead, thus freeing Li+ as the conducting species and resulting in almost unity t+.