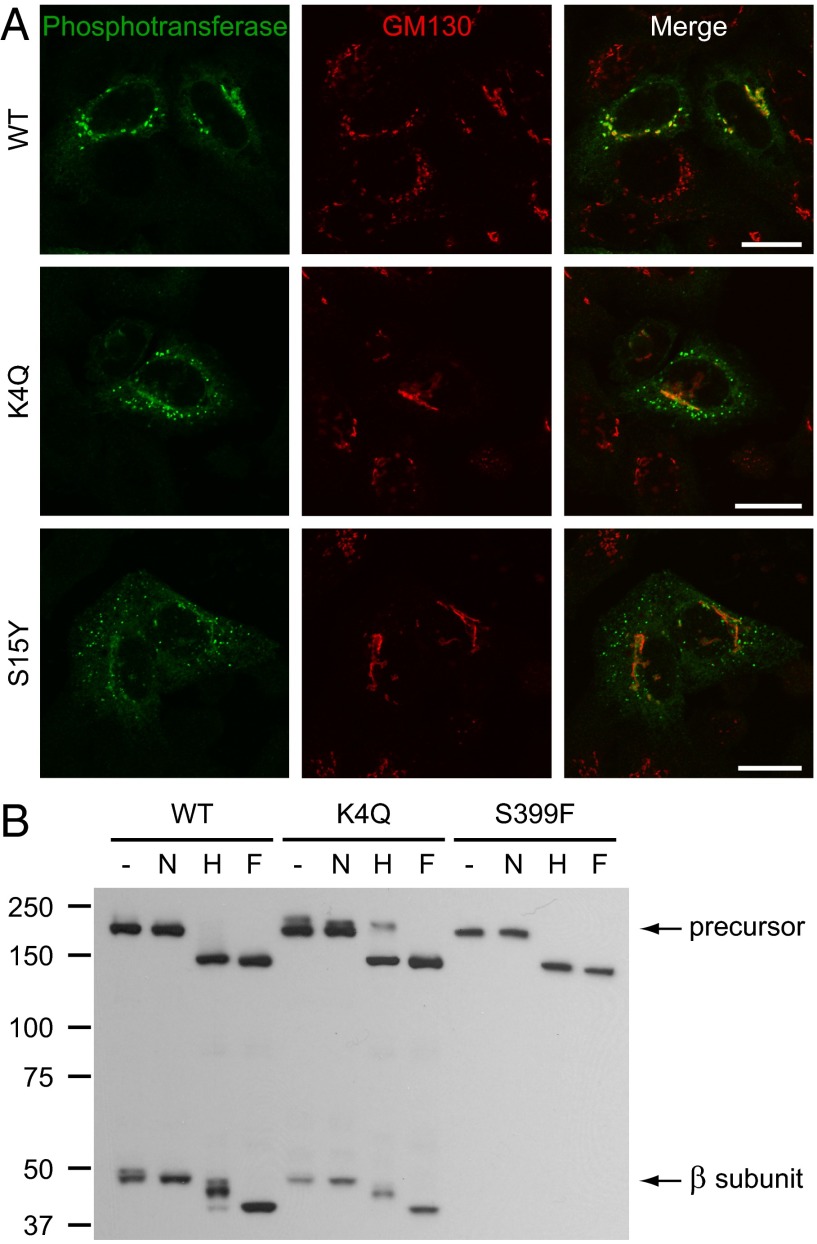
Fig. 2.
K4Q and S15Y αβ phosphotransferase mislocalize in punctae that represent post-ER compartments. (A) Immunofluorescence staining for phosphotransferase (anti-α subunit) and the cis-Golgi marker GM130 in HeLa cells 16 h after transfection with WT, K4Q, or S15Y αβ phosphotransferase. Although the WT enzyme localizes to the Golgi complex, the mutants localize in punctae with only low levels in the Golgi. (Scale bars, 20 μm.) (B) Western blots (anti-V5 antibody) of transfected HEK293 cell lysates treated with neuraminidase (N), Endo Hf (H), or PNGase F (F) (3 h at 37 °C) or untreated (−) show partial digestion of the β subunits (WT and K4Q) by Endo Hf. Although the WT αβ precursor almost completely shifts to ∼150 kDa after digestion by Endo Hf, the K4Q precursor is partially insensitive to Endo Hf (∼210-kDa band) and shows a clear shift after neuraminidase treatment, indicating the presence of complex-type N-linked glycans.