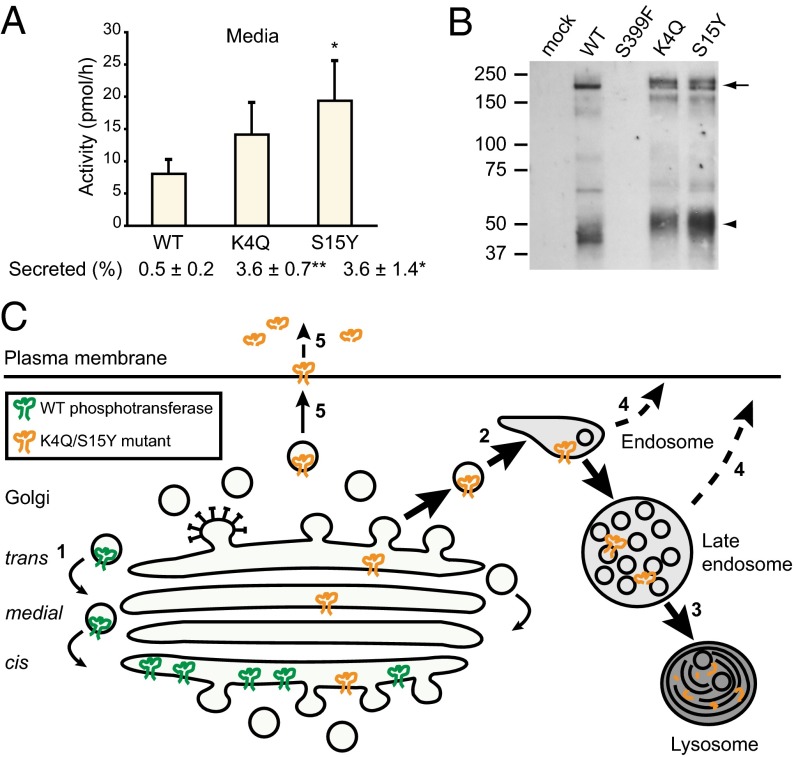
Fig. 6.
K4Q and S15Y αβ phosphotransferase are released into the media. (A) The activity of phosphotransferase toward α-MM was measured 48 h after transfection in HEK293 media after a 15-h incubation. The percentage secreted represents the total activity in the medium as a percentage of the total activity in cells and medium. See Fig. S5A for the activity in the cell lysates. Data are average values of 3 independent experiments, error bars represent SD. *P < 0.05; **P < 0.01 (Student t test). (B) Western blots (anti-α subunit antibody) of media from transfected HEK293 cells collected after a 6-h incubation. One-fifteenth of the total volume of medium was loaded for K4Q; all other media volumes were adjusted relative to the protein concentration of the cell lysates (see Fig. S5B for Western blot on cell lysates). The arrow indicates the αβ precursor/α subunit, and the arrowhead is a putative degradation product of the α subunit that was not observed in the cell lysates. (C) Model for the mislocalization of K4Q and S15Y αβ phosphotransferase. After synthesis and folding in the ER, the WT precursor is transported to the cis-Golgi, where it is activated by proteolytic cleavage into α and β subunits. Gradually, the enzyme progresses to the medial and trans cisternae, where it acquires complex-type N-linked glycans, but it is then recycled to the cis-Golgi (1), where the bulk of the enzyme is localized at steady state. The K4Q and S15Y mutants are transported from the ER to the Golgi complex at the same rate as the WT enzyme but fail to be retained in the Golgi. Rather, the mutants pass through the Golgi and undergo transport to endosomes (2) and lysosomes, where they are degraded (3). Some of the mutant enzyme is released into the extracellular milieu, either directly from endosomes (4) or via the plasma membrane (5).