Fig. 6.
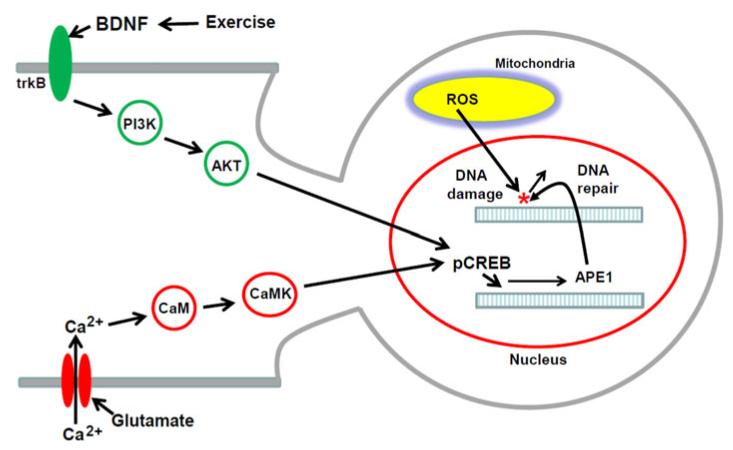
Model for the mechanism by which BDNF enhances repair of oxidative DNA damage in neurons. Neurons generate considerable amounts of reactive oxygen species (ROS) in mitochondria (superoxide and hydrogen peroxide) which can cause oxidative damage to DNA bases. Under usual conditions, neurons express sufficient amounts of the base excision repair (BER) enzymes that repair oxidative DNA lesions (OGG1, APE1, DNA ligase and DNA polymerase β). However, when levels of excitatory synaptic activity are elevated, or during injury, aging, and neurodegenerative disease, basal levels of repair are insufficient to prevent excessive accumulation of DNA lesions. Activation of BDNF receptors (TrkB) results in activation of PI3 kinase (PI3K) which phosphorylates AKT. AKT, in turn, phosphorylates the transcription factor CREB (pCREB), which then binds to the promoter of the APE1 gene to induce the expression of APE1 and enhance BER. In addition, Ca2+ influx through ionotropic glutamate receptor channels can upregulate APE1 expression via a pathway involving calmodulin (CaM) and calmodulin-dependent protein kinase (CaMK) which phosphorylates and activates CREB (13). By upregulating APE1 and BER, environmental factors that induce BDNF expression, such as regular exercise, may increase the resistance of neurons to oxidative DNA damage