Abstract
Many genetic and environmental perturbations lead to measurable changes in bone morphology, matrix composition, and matrix organization. The skeletal system is highly adaptive, such that genetic and environmental perturbations affecting one trait are often accompanied by compensatory changes in other traits. This adaptation should be considered when attempting to draw meaningful conclusions about the role of a gene, for example. The challenge is to figure out which alterations can be attributed to the perturbation and which result from adaptive changes associated with establishing mechanical function. The goal of this chapter is to describe straight-forward biomechanical methods that can be used to determine whether a genetic or environmental perturbation affected bone strength. We introduce a systematic method for evaluating how bone strength was altered in the context of morphology and tissue-level mechanical properties, which are determined in large part from matrix composition, matrix organization, and porosity. We present this work as a first step toward screening mice for a phenotypic effect and for establishing the associated biomechanical mechanism by which function was altered. The outcome of these analyses generally provides insight into the next set of experiments required to further connect the cellular perturbation with the functional changes. The protocols were written to enable researchers without a background in engineering to conduct the assays or to enable researchers to better understand the outcomes of similar assays conducted by colleagues knowledgeable in engineering.
Keywords: bone, biomechanics, strength, nanoComputed Tomography, cortical bone, trabecular bone, adaptation
Introduction
Many genetic and environmental perturbations can affect the skeletal system. These perturbations often lead to measurable changes in bone morphology, matrix composition, and matrix organization. Many investigations stop here, drawing conclusions about the role of a gene, for example, based on these structural and compositional alterations. However, these associations are only revealing part of the story. First, it is important to recognize that many perturbations affect body mass. Adjusting traits for body mass is essential, because bigger mice generally have bigger bones. The challenge is to determine whether changes in a trait are greater (or less) than expected for the effect on body mass. Second, to determine whether a genetic or environmental perturbation also affected bone strength requires additional knowledge of the complex adaptive nature of the skeletal system.
The goal of this chapter is to describe straight-forward biomechanical methods that can be used to determine whether a genetic or environmental perturbation affected bone strength. We introduce a systematic method for evaluating how bone strength was altered in the context of morphology (bone size and shape) and tissue-level mechanical properties, which are determined in large part from matrix composition, matrix organization, and porosity. We present this work as a first step for screening mice for a phenotypic effect and for establishing the associated biomechanical mechanism by which function was altered. Additional testing is often required (e.g., FTIR, Raman, Nano-indentation) and there are many resources that can help with these methods. The protocols were written to enable researchers without a background in engineering to conduct the assays or to enable researchers to better understand the outcomes of similar assays conducted by colleagues knowledgeable in engineering.
Basic Protocol 1: BONE HARVESTING PROTOCOL
Harvesting bones is relatively simple, but must be done without breaking them. This requires a basic understanding of anatomy to disarticulate and extract a bone without damaging the epiphyses, which can easily be sheared off in younger mice.
Materials
Mouse
Absorbent pads
2ml Eppendorf tubes
1X Phosphate Buffered Saline (PBS)
10% Neutral Buffered Formalin (10% NBF)
Tissue cassettes (Fisher Scientific, Histocassette II)
Permanent marker
No. 2 pencil
Freezer storage boxes, cardboard or plastic
1 liter wide mouth HDPE bottles (Qorpak)
Gloves
Safety goggles
Gauze
Prepare sample containers
-
1
Label cassettes for bones going to histology with the No.2 pencil. Write the animal identification number and the bone on the each cassette in two places, in case one rubs off or gets smudged.
Never use pen or permanent marker, as the chemicals used for plastic or paraffin embedding will remove the label and you will have no way to identify your specimens! Entire research studies have been lost because of labeling with pen. Do not let this happen to you! -
2
Label 2ml Eppendorf tubes for bones that will be imaged and/or used for mechanical testing using permanent marker. Write the animal identification number and bone on the top and side of each tube. See Figure 1 for an example of proper labeling of an Eppendorf tube and tissue cassette.
-
3
Label freezer storage boxes using permanent marker. Write the animal identification numbers (can group, i.e., B6 1–10, as opposed to B61, B62, B63…). Indicate the type of bone
We separate bones by type, so femurs would go into one box, spines would go into a second box, etc. -
4
For every 20 cassettes, fill one of the 1 liter bottles with 10% neutral buffered formalin, or a fixation agent that works with your histological processing method.
Wear gloves and safety goggles. Bottles should be filled under a fume hood.Place the 1 liter bottles on an absorbent pad.Fill 1 liter bottle from the container of 10% NBFAffix a permanent label to the outside of the bottle that includes: 10% NBF (or other chemical, if used), animal identification numbers, and bone typeTightly close the bottle until ready to use it.We keep our bones separated by type, so femurs would go into one bottle, spines would go into a second bottle, etc. -
5
Prepare gauze by cutting it into long pieces that will wrap around an entire bone and soaking it in PBS
Fig. 1.

Harvest bones
-
6
Sacrifice the mouse, according to your protocol and institutional guidelines. Be sure to collect the body mass of each mouse before starting dissection.
-
7
For bones that will be imaged or mechanically tested:
Remove as much soft tissue from the bone as possible with gauze. If using a scalpel, be careful not to cut or scratch the bone surface.Wrap the bone completely in PBS-soaked gauze.Place the bone in the appropriately labeled Eppendorf tube. Close the tube so it is sealed.Place the Eppendorf tube in the appropriately labeled freezer storage boxWhen done, place all freezer storage boxes in a −20°C or −40°C freezer.
Alternatively, instead of wrapping bones in PBS-soaked gauze, place the bone in the Eppendorf tube and then fill the tube most of the way with PBS. Leave room for the PBS to expand with freezing, but make sure the entire bone is covered with PBS before freezing.
-
8
For bones that will be processed for histology
Remove as much soft tissue from the bone as possible with gauze, being careful not to disrupt the periosteum.Place bone in the appropriately labeled histology cassette.Place the cassette in the appropriately labeled bottle of 10% NBF (or other fixative)When done, put all 1 liter bottles in an area where they are away from direct sunlight and out of heavy travel areas. Ensure any shelves used are sturdy enough to support the weight, especially if there are many bottles.
Support Protocol 1: MMA SOLUTION PROTOCOL
Safety guidelines
Wear goggles, gloves and lab coat.
Execute under the fume hood.
DO NOT use a metal scoop when weighing Benzoyl Peroxide, it may cause a spark. Instead, use a wooden spatula.
Use caution when transporting Benzoyl Peroxide and DO NOT breath the fumes
Materials
Methyl Methacrylate (MMA)
N-Butyl Phthalate (n-BP)
Dry Benzoyl Peroxide (if using hydrated, add 25% more by weight)
1–1000mL graduated cylinder (designated for MMA)
2 funnels (one labeled methyl and one labeled butyl)
1–250mL graduated cylinder (designated for MMA)
4 weigh boats
4 – 1 liter wide mouth HDPE bottles (Qorpak)
1 wooden stick
Shaker (Orbital Shaker)
Gloves
Goggles
Lab coat
Prepare MMA
Label 1 liter bottles with permanent marker. Bottles should be labeled: MMA I, MMA II, MMA III, and MMA IV.
Place 765mL MMA in each of the four bottles
Add 140mL n-BP to each of the four bottles
MMA I is complete once placed on the shaker for 1 hour, store at room temperature in a flammable cabinet.
Weigh 9.0g dry benzoyl peroxide. Place this in MMA II. Mix on shaker for 1 hour, store at −20°C.
Weigh 16.75g dry benzoyl peroxide. Place this in MMA III. Mix on shaker for 1 hour, store at −20°C.
-
Weigh 17.75g dry benzoyl peroxide. Place this in MMA IV. Mix on shaker for 1 hour. Thicken in 37°C water bath. Store at −20°C.
Thickening should be done carefully. Usually this process will take a few days but each solution behaves a little differently. Constantly check the solutions progress, no less than every 30 minutes and shake well. A good indication that the process is beginning to speed up is when the bottle begins to contract and the solution turns slightly yellow. At this point begin monitoring it more frequently. Continue this process until it has a consistency similar to honey.If the reaction heats up too quickly it may explode. If at any point the reaction proceeds too quickly, the MMA bottle can be placed in −20°C to cool it off and pause the polymerization process.DO NOT open any MMA bottle until thawed and warmed to room temperature. It is important to avoid introducing water into the solution, as it can mess up the embedding process.
Basic Protocol 2: PLASTIC EMBEDDING PROTOCOL
Bone samples can be embedded in plastic or paraffin to evaluate changes in matrix organization arising from a genetic or environmental perturbation. Histomorphometry is an additional analysis that can be done to provide information on changes in bone formation rates that may account for any structural alterations associated with a genetic or environmental perturbation. To conduct histomorphometric analyses, bone samples have to be embedded in plastic without prior decalcification. This is a two-step embedding process, which helps to best center the bone in the plastic cylinder.
The bone fluorescent labels used to mark new bone deposition are incorporated into the mineral. These labels will be removed upon decalcification. There are many fluorescent labels available and these can be chosen based on the type of filters available in the epiflourescent microscope. We inject each mouse with 30 mg/kg of mouse body weight of Calcein at 7 days prior to sacrifice and then with Alizarin-red at 2 days prior to sacrifice, both at a concentration of 10mg/mL. This puts 5 days between labels; this injection protocol is sufficient for mice less than 12 weeks of age. After 12 weeks of age, bone formation has slowed quite a bit so the time between injections can be increased to make sure there is sufficient space between the labels to generate accurate measures of mineral apposition rate.
Materials
Tissue cassettes (Fisher Scientific, Histocassette II)
10% Neutral Buffered Formalin (NBF)
1 liter wide mouth HDPE bottles (Qorpak)
70% Ethanol
1X phosphate buffer solution (PBS)
Distilled water
Villanueva Osteochrome Bone Stain (Polysciences, Inc.), optional
100% Methanol
Ethylene glycol monoethyl ether (EGME)
Methyl Salicylate, 99%
Methyl Methacrylate - MMA I, II, III and IV (See chemical protocol)
5mL plastic vials
Forceps
Vacuum Oven (Fisher Scientific Isotemp or Eurotherm 91e Blue)
Diamond wafering blade (e.g., Isomet Low Speed Saw; Buehler, Inc., Lake Bluff, IL USA)
600–1000 grit sandpaper
1″ × 3″ plastic slide
Cyanoacrylate
Light microscope (Zeiss Axioplan2, Carl Zeiss IMT Corp., Minneapolis, MN, USA)
Small drill
Small wooden dowel rod
Prepare specimens
See the chemical preparation protocol (Support Protocol 1) for specifics on making chemicals involved in this process. Harvest bones according to harvesting protocol (Basic Protocol 1).
Fixation
-
1
Submerge specimens, in cassettes, in NBF for 24–48 hours at room temperature.
-
2
The samples may be left in NBF at room temperature for short term storage but should be moved to 70% ethanol after no more than a few months.
-
3
When ready to continue to the next step, wash twice in PBS or water for 12 hours each at 4 deg C.
Pre-stain using Villanueva Bone Stain (Osteochrome) - Optional
-
4
Soak in the stain for 48 hours
-
5
Wash in 100% Methanol, twice for at least two hours each to remove any excess stain.
Dehydrate
This portion of the process can be automated if there is access to a programmable plastics processing system. In a processor the total time for this section is 48 hours.
-
6
Pre rinse with EGME. If coming from NBF, rinse for at least 12 hours. If coming from ethanol this time can be shortened.
It is important from this step forward to avoid any introduction of water into the system. -
7
Perform dehydration in four changes of EGME, using separate bottles labeled 1 thru 4. Start by placing the specimens in bottle 1 for 4 hours then move to bottle 2 for 4 hours and so on.
To save chemicals, after dehydrating approximately 20 bones, rotate bottle 2 to bottle 1 then bottle 3 to bottle 2 and so on, only replacing bottle 4 with new EGME. Continue this rotation process. The logic here is that the first change of EGME will be contaminated the most with ethanol. -
8
Rinse in 3 changes of 2-propanol, 4 hours each.
-
9
Clear using two changes of methyl salicylate for 4 hours each change.
Employ rotation method again to save chemical costs. -
10
Finally process in MMA I for 12 hours.
Embed specimens
Infiltration
-
11
Submerge in MMA I for at least 48 hours. If desired, place on medium speed agitator to increase infiltration effectiveness.
-
12
Submerge and agitate in MMA II for at least 48 hours. This step should be done in a refrigerator.
DO NOT open the MMA II until it has come to room temperature. Water vapor can condense on the surface of cold MMA when exposed to normal, humidified air. This will introduce water into the solution and compromise the embedding procedure. -
13
Submerge and agitate in MMA III for at least 48 hours, this step should be done in a refrigerator.
DO NOT open the MMA III until it has come to room temperature.
Create base for embedding
-
14
Allow MMA IV to thaw and come to room temperature prior to opening the bottle.
-
15
Fill each vial, one for each sample, just slightly less than half way with the MMA IV. Spread the plastic along one side of the tube vertically creating a half cylinder.
-
16
Tighten caps on the vials and transfer to a 37deg C oven for hardening. This step will take 1–2 days.
Be sure to place the vials in the oven on their sides but preventing them from rolling, so that the result is just less than a half cylinder of hardened plastic. Also leave space between the curing vials for air circulation.
Sample placement
-
17
Add MMA IV and samples to bases. Attempt to center and orient the bone with the vertical axis of the vial as best as possible. It is ideal to place the anterior surface of the bone, being the flattest side, on the hardened plastic half.
To remove bubbles, place vials in vacuum on sides with caps off for 10 minutes. Bubbles should rise out of the sample at this time, which will result in a translucent plastic. -
18
Store the full vials at room temperature for 48–72 hours with caps tightly on to continue the polymerization process while preventing any further bubble formation.
-
19
Transfer to a 37deg C oven for final hardening. To ensure that the entire sample is hardened, leave in this environment for 3–4 days.
Sectioning
-
20
Bone samples can be sectioned using any commercially available metallurgical cutting system with a diamond wafering blade.
-
21
Carefully remove the sample from the plastic vial. This can be done by drilling a small hole in the bottom of the vial and gently pushing the sample out using a dowel.
-
22
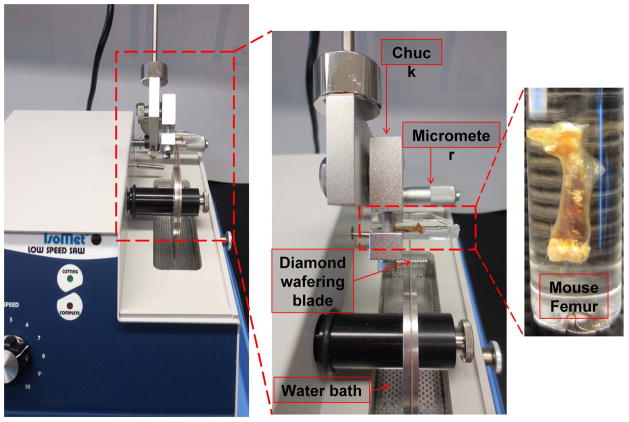
Use a chuck, usually supplied by the manufacturer, to hold the sample in place while it is being sectioned (Fig. 2).
-
23
Adjust the orientation of the sample so the blade sections the bone transversely.
-
24
For mouse femurs, cut the bone just distal to the third trochanter. We have found that 200 micron thick sections are easily handled. If the section is too thin, then routine handling can result in the section being flexed which often results in cracking of the bone sample.
-
25
Repeat sectioning 3–4 times or until there are a sufficient number of sections to represent the mid-shaft.
-
26
The sections can be lightly polished on one side using 600–800 grit sandpaper. This side can be glued to a 1″ × 3″ plastic slide using cyanoacrylate.
-
27
The slide should be ground and polished using a series of sandpapers (600–1000 grit) followed by a series of diamond slurries (10 micron, 6 micron, 1–2 micron). The final sample thickness should be approximately 30–50 microns
-
28
View the section using a light or epifluorescent microscope.
Fig. 2.
Basic Protocol 3: COMPUTED TOMOGRAPHY PROTOCOL
To determine whether a genetic or environmental perturbation affected bone morphology, bone samples can be imaged using micro Computed Tomography. There are many systems on the market and they all generate 3-dimensional images from which cross-sectional morphology of cortical bone and trabecular architecture can be measured. We present protocols for a nanoCT. The procedures are similar to those employed in microCT units except the nanoCT can be used to acquire images with resolutions approaching 0.5 microns. This high resolution may be useful when assessing morphological properties for young mice, which have small structures with low mineralization levels. This combination of traits makes it difficult to acquire high fidelity images using conventional microCT systems.
The general concepts presented below can be adapted to other computed tomography systems. However, with closed tube systems it is not optimal to push the system to its limits because it decreases the overall lifetime of the system. Open tube systems allow more parameter flexibility and range because maintenance on the system is easy and relatively inexpensive.
Materials
Nanotom S (GE Sensing & Inspection Technologies GmbH) (Fig. 3)
Filter material (0.25mm Aluminum, 0.3mm Aluminum, 0.762mm Aluminum)
1X Phosphate buffered saline (PBS)
Cylindrical specimen tube (made out of acrylic, polycarbonate, or other similar material)
Bone holder (made out of acrylic, polycarbonate, or other similar material)
Latex rubber band
Acrylic equilibration bath
Custom fixtures to hold specimen holder and acrylic equilibration bath (if needed, will depend on your design)
Calibration standard
Acrylic pieces
1.69mg/cc cortical bone equivalent rod – hydroxyapatite standard (Gammex)
Computers for acquisition and reconstruction (128GB RAM; 8 CPUs; Intel Xeon 3.2 GHz; NVIDIA Graphics cards; 2TB hard drive; Linux virtual machine)
VGStudio MAX 2.1 Software (Volume Graphics GmbH)
MicroView Advanced Bone Analysis (v. 2.2; GE Healthcare)
Fig. 3.
Design fixtures for imaging system
-
1
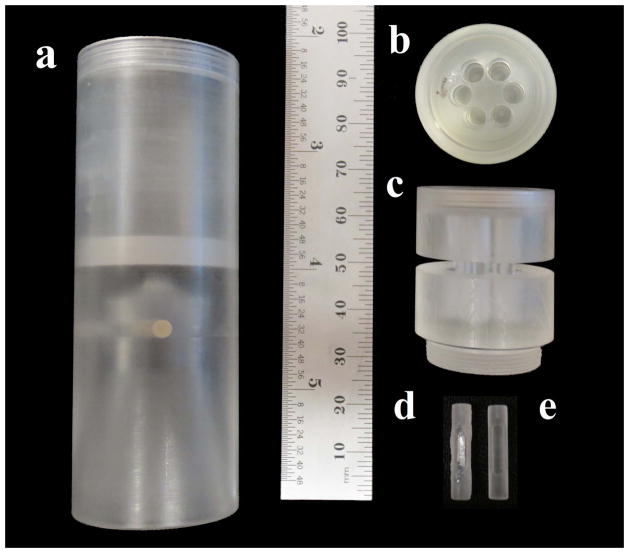
Design a cylindrical tube that can be placed on the sample stage with no movement (Fig. 4a). The tube must also be able to contain water or PBS without leaking and contain a specimen holder with the bones in their individual places during the scan (Fig. 4b and Fig. 4c). A great option for material is acrylic because it has similar attenuation characteristics as water. If using acrylic be aware that alcohols can cause the material to crack and eventually shatter. Polycarbonate is an alternative option.
-
2
Design any necessary fixtures needed to hold the sample holder to ensure alignment relative to the beam and detector.
-
3
Design a calibration standard that can be incorporated in the sample holder.
The calibration standard utilized in this protocol consists of a region of air and water and a beam of a 1.69mg/cc hydroxyapatite mimicker (Fig. 4d). This standard is placed in one well of the specimen holder. A quick setting plastic can be used to seal the hydroxyapatite beams into the machined calibration standard (Fig. 4e). -
4
Incorporate an “equilibration bath” into the system to improve signal quality. This bath is an acrylic block with a central hole (Fig. 5). The cylinder holding the samples slides into this central hole. The equilibration bath creates a uniform thickness of material (acrylic, water) to improve uniformity of the X-ray beam.
-
5
Eliminate all metal. Sonicate all pieces that will be visible in the scan. Avoid the use of metal utensils when placing the sample in the holder or manipulating the holder.
A small piece of metal can essentially compress all the other signals in the sample into a much smaller range of grey values, resulting in decreased bone density detectability.
Fig. 4.
Fig. 5.
Prepare specimens
-
6
Fill the holder and tube with water, PBS, or other solution prior to inserting the individual samples into the cylinder. This is necessary to avoid bubbles that affect scan quality.
-
7
Secure each bone sample in a well of the cylinder with a latex rubber-band.
Acrylic pieces of varying size and shape are helpful when stabilizing specimens varying in size.Large bubbles scatter the X-ray beam and cause hardening of the X-rays which can lead to non-uniformity in the reconstructed image field.
System settings
-
8
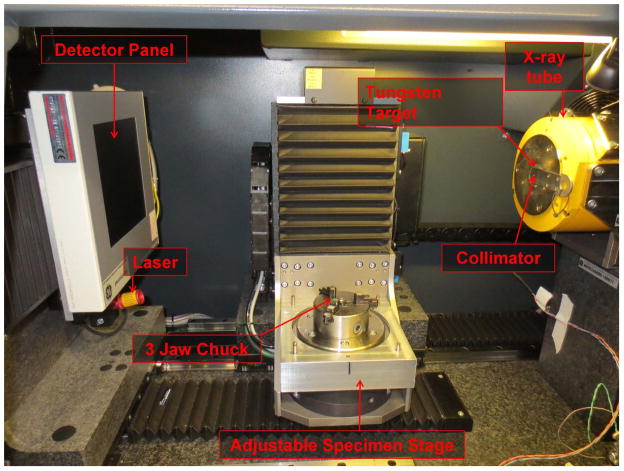
Use a Tungsten target for imaging bones because a higher quality, higher energy X-ray beam is produced. (Fig. 6)
Molybdenum is also a good target option for CT systems and can be used for imaging materials with lower levels of mineralization. -
9
Employ an aluminum filter of 0.3mm when imaging mouse bones. The exact thickness depends on the overall specimen and system configuration.
A filter helps to eliminate low energy X-rays and minimize beam hardening artifacts. -
10
Manipulate the axes to get the specimen into view. Visually check for air bubbles or metal particles that may have been missed. The bubbles appear very light gray, due to little attenuation of the X-ray beam. Metal particles appear sharp and black, because they highly attenuate the beam.
-
11
Adjust the voltage and current settings of the system. These adjustments are useful for increasing image quality, detail and contrast, depending on the specimen, solution and holder. Higher voltages are used to increase X-ray penetrating power, therefore if the sample being imaged is dense or thick, increasing this setting may be necessary.
Be aware that increasing this value too much on a sample that is not very dense or large will decrease image quality substantially because the X-ray may just pass right through the bone without being attenuated.We have found that 85kV works best with our set-up. -
12
Higher current settings are used to increase the number of X-rays reaching the sample. Therefore more X-rays will be exiting the sample and reaching the detector. The result is a wider range of grey values and greater detail detectability.
Keep in mind that increasing this setting too much will spread out the background peak therefore decreasing the signal to noise ratio. Furthermore, radiation deposition into a sample is highly correlated with current, therefore increasing the current increases the amount of radiation that the specimen will receive.We have found that 220 μA works best with our set-up.
Fig. 6.
Watch the overall power settings on the system. Do not establish settings that are outside the acceptable power range of the system. Ignoring the systems maximum power settings will greatly decrease the overall lifetime of the system and its components.
-
13
Change the timing. Increasing the timing, increases the exposure of the detector to the X-ray beam for each image increasing image quality.
Increasing timing comes at a cost, because it significantly increases the scan length.We have found a timing of 2000ms to be best for decreasing graininess, or noise, but not compromising scan time. -
14
Select a suitable averaging value to maximize image quality without compromising scan time.
Averaging specifies the number of images taken at each angle and integrating these images to increase the signal to noise ratio. Higher averaging decreasing the noise in the image but increases the scanning time substantially.We have found an averaging of three to be adequate. -
15
Adjust the skips. Skips greater than one are generally used for very long, high power scans to correct for detector afterglow.
Again, this comes at a cost, longer scan times.We have found skipping one frame to be adequate. -
16
Establish an observation ROI. This is a further correction of background signal. When choosing the representative ROI, ensure that no part of the sample passes through the region during the scan.
Running the scan
-
17
Run bright and dark field (gain and offset) images using the scanning parameters.
Replace sample holder with a tube of water if the sample is being scanned in water. If the sample is being scanned dry, calibrate with nothing in the way of the beam.Turn the cabinet light off during calibration and during the scan. The detector in the Nanotom S model can detect the cabinet light, resulting in slightly shifted grey values. If using another system that has an inside cabinet light, verify with the system manufacturer or perform an onsite validation study. -
18
Replace the sample and start the scan.
Reconstruction
Each lab may use different reconstruction software. We describe a general protocol that can be applied to most reconstruction software in a stepwise fashion.
-
19
Select the region to reconstruct. If your specimen(s) do not take up the entire volume, it is optimal to select a sub-volume to decrease the reconstruction file size, as they can be very large with the nanoCT.
-
20
Correct for center of rotation errors in the scan.
-
21
Compare the first and last image verifying there is no visible movement during the scan. If there was no movement the difference between first and last images should be uniformly grey.
If there is movement, correct by shifting the X and Y pixels. If the movement is uncorrectable, it will be necessary to reposition the sample and repeat the scan.If there is movement of air bubbles or packing material around the sample but no movement of the sample, then do not correct the shift. Correction for movement in background will apply an unwanted shift to the specimen, resulting in decreased image quality of the specimen. Furthermore, air bubble movement during a scan is extremely difficult to correct. -
22
Perform a median filter, ROI-CT filter and utilize the observation ROI to correct for variation in background grey values that occur during the scan.
Some systems do not allow the selection of an observation ROI. -
23
Save the reconstructed file.
-
24
Follow the above procedure to reconstruct individual volumes of the calibration standards (i.e. air, water and HA) as well.
Scaling to Hounsfield (HU) and isolating individual bone volumes
This protocol has been specifically established for use with VGStudio MAX 2.1, software that was obtained with our scanning system, the Nanotom S.
-
25
Import the .vgi file of interest.
-
26
Record the file dimensions, x, y, and z. These will be necessary to import the image into MicroView for analysis.
-
27
Change the file type to signed 16-bit.
-
28
Select the histogram button, then the calibrate tab. Convert the grey values from the image acquisition to HU values. Do so by entering the grey value from the air standard into the background box and mapping that to −1000.0. Then enter the grey value of the water standard in the material box and map that to 0.0. Apply these changes.
When choosing the regions of standard to calculate the average grey value, be sure the region is not affected by any artifacts, i.e. Beam hardening, shadowing, bubbles etc. -
29
Select the preview button. Isolate the single bone of interest (Fig. 7) by drawing an ROI using the four toggle tabs. This is done to reduce overall file size and load time in the analysis software. The files generated are still very large.
-
30
Press the finish button. The file will load in Volume Graphics.
-
31
Go to the file tab and export the file as a raw data set. Use the histogram in Volume Graphics to confirm that the file has scaled appropriately.
Fig. 7.
Image analysis
Import file into MicroView
-
32
Select the .raw file.
-
33
Enter the x, y and z voxel size.
-
34
Select raw and 3D image boxes.
-
35
Change the file type to little endian and short.
-
36
Enter the x, y, and z file dimensions.
Calibration standard analysis
-
37
Open the water volume. Select the largest possible ROI containing only water. Find the mean grey value for the volume selected and record this as the arbitrary density unit (ADU) for water.
-
38
Open the hydroxyapatite (HA) volume. Select a ROI that maximizes coverage of the sample but stays central enough to avoid any partial volume effects felt by the edges. Find the mean value for the volume and record this as the ADU for HA.
It is a good idea to analyze the HA for density and mean grey value as a way to verify that there is no drift in the system scanning over time. -
39
Reorient each bone volume so the long axis of the bone is parallel to the z-axis.
Regional bone analysis for mid-shaft of the femur
-
40
Open the reoriented file.
-
41
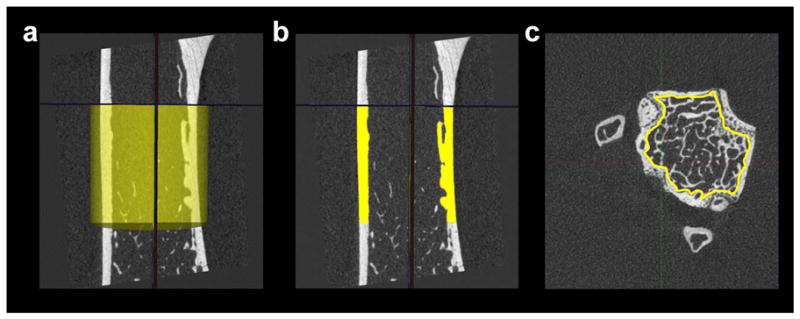
Select a 2.5mm region of the bone, using a cylindrical ROI, just below the third trochanter extending distally (Fig. 8a). Be sure to include both bone and non-bone regions.
This specific site is preferred because there is little trabecular bone that will be included during thresholding and it allows for consistency throughout analysis. Further, this region is generally where the sample fails during the whole bone 4-point bending tests. -
42
Auto threshold the region.
We prefer to threshold samples individually, rather than apply a single threshold value for all samples being studied. Variation in scanning parameters or even mineralization of the sample itself can affect the threshold value. A blanket-threshold value could lead to decreased cross-sectional area values for bone samples with lower mineralization and increased cross-sectional area values for bone samples with higher mineralization. -
43
Segment the cortical regions of the bone (Fig. 8b).
-
44
Measure bone mineral content (BMC), bone mineral density (BMD), tissue mineral density (TMD), cortical area (Ct.Ar), moments of inertia (Imax, Imin, J), cortical thickness (Ct.Th), marrow area (Ma.Ar) and total area (Tt.Ar)
Fig. 8.
Regional bone analysis for distal femur trabecular bone
-
45
Load the reoriented image and perform a median filter if the image looks fuzzy.
-
46
Rotate the bone so that the distal portion is on top. Scroll through slices until just under the growth plate.
-
47
Use the spline feature to create an ROI by outlining the trabecular bone, getting as close to the cortical shell as possible (Fig. 8c). Repeat this for a fixed proximal distance, skipping every 10 slices. For any study, the length of the image volume should be based on a fixed distance (e.g., 2mm) or as a percentage of bone length. This decision is based on acquiring volumes of trabecular bone that best represent the scientific question.
-
48
Save the contours of this ROI, interpolate, and generate a 3D ROI.
-
49
Auto threshold.
-
50
Enter the HA ADU and the water ADU determined during the calibration standard analysis.
-
51
Measure BMC, BMD, BV/TV, connectivity of the trabecular structure, trabecular plate thickness, trabecular plate number and trabecular plate separation.
Regional bone analysis for vertebra trabecular bone
-
52
Load reoriented files.
-
53
Use the transverse view to analyze the image. Scroll to the most distal end of vertebra, just past the growth plate.
-
54
Select spline, outline trabecular bone, get as close to the cortical shell as possible because the contours will also be used later for cortical analysis. Also be sure not to include any of the processes in the trabecular contours.
-
55
Spline through each slice until the proximal growth plate is reached.
-
56
Save the contours then click interpolate and generate 3D ROI.
-
57
Verify that everything looks appropriate on the 3D ROI before moving on.
-
58
Save the crop region containing only trabecular bone.
-
59
Open the cropped trabecular file and apply a median filter if the image looks slightly fuzzy.
-
60
Auto threshold.
-
61
Measure the same traits as in the distal femur section.
Regional bone analysis for vertebral cortical bone
-
62
This method is the same as the mid-shaft femur protocol except open the file and load the same contours used to isolate the trabecular areas.
Basic Protocol 4: WHOLE BONE 4-POINT BENDING TESTS
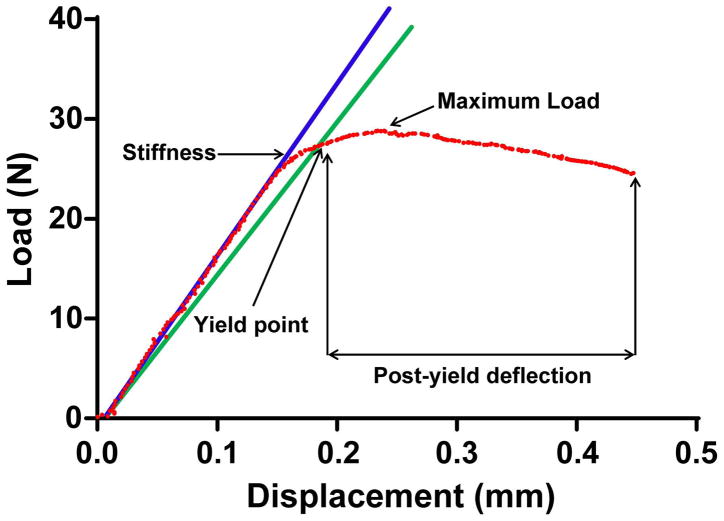
Whole bone 4-point mechanical testing can be used to quantify mechanical properties of the cortical bone. These properties include stiffness, maximum load, post-yield deflection, and work-to-fracture. This protocol will test the bones to failure, so if you need intact bones for other protocols (i.e., imaging), make sure to complete those protocols before doing mechanical testing.
Before conducting this protocol, you should be familiar with how to operate your mechanical testing system. There are several companies that manufacture high quality testing systems; each operates slightly differently in terms of software and hardware. The steps below will be defined in general terms, to allow for these differences. For this protocol, a mechanical testing system that has an actuator travel of ±2 inches, can achieve a testing rate of 0.05 mm/sec, and a sampling frequency of 25 Hz is needed. You will need to record the test data, specifically the test time, displacement, and load.
The 4-point testing fixture should have loading points that are a set distance apart. The two points on the bottom are 6.5 mm apart. The two points on top are centered relative to the bottom points, and are 2.2 mm apart. There will need to be some adjustability in the vertical direction of one loading point so that variation in bone width can be taken into consideration. This ensures that all four loading points engage the bone at the beginning of the test. In some setups the bottom points are adjustable in the vertical direction independent of each other; in other systems the two bottom points are machined to move as one unit. Adjustability as a unit or individually in the bottom two points is a matter of personal preference.
The protocols below detail mechanical testing of mouse femora. These protocols can also be used for mechanical testing of mouse tibia and metatarsals, although the distances between the points may have to be modified, depending on bone length.
Materials
Mechanical testing system (Fig. 9a, 9b)
4-point fixtures (Fig. 9c)
Pick-ups
Glass petri dish
Absorbent pads
Small diameter (roughly 2mm) wooden dowels cut into 1″ lengths
1X Phosphate Buffered Saline (PBS)
Gauze
Laboratory notebook
Fig. 9.
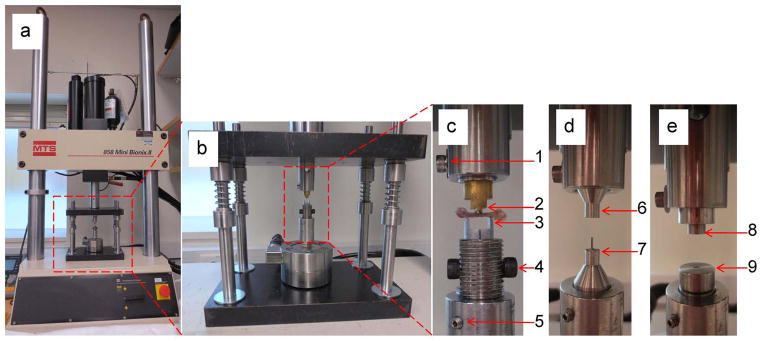
Testing preparation
-
1
Remove bones from the freezer. Allow them to thaw for an hour before testing.
Make sure the bones are completely thawed before testing.Measure the length of the bones before testing. -
2
Warm up the mechanical testing system according to the manufacturer’s guidelines.
We run our hydraulic system for 15 minutes, cycling ±5mm. -
3
As the system is warming up, set up the testing area.
Place an absorbent pad by the testing system where the preparation of the bones will take place. On the pad, place the bottle of PBS, gauze, wooden dowel rod sections, pick-ups, and a glass petri dish, with PBS in itFigure out a method so that it is known which bones have been tested, and which still need to be tested. Generally, having two separate areas or containers, one for tested bones and one for untested bones, works best. -
4
After system warm up is complete, set up the system so that the test will run at a displacement rate of 0.05mm/sec. The data should be recorded at 25Hz. If able, set a distance limit so that there is no danger of yielding a load cell if the bone does not break.
Testing
Test standards
-
5
Place a 12 mm section of wooden dowel in the 4-point testing fixture.
-
6
Adjust the top two points so they just contact the dowel rod.
-
7
If needed, adjust the fixture so that both bottom points of the fixture are now in contact with the dowel rod. For our set-up, one of the bottom points is adjustable in height. We will leave one point set, and adjust the other slightly if needed to ensure all four loading points touch the sample prior to testing.
-
8
Begin a test while recording the load and deflection data. Pay close attention to the testing fixtures, so that the actuator can be stopped as soon as the dowel rod has broken.
Do not let the top of the testing fixture come into contact with the bottom of the testing fixtures! This will yield the load cell, causing permanent damage. This can be avoided by building an emergency stop button into the software or using the systems’ physical emergency stop button. -
9
Bring the actuator back up to the starting point.
-
10
Test enough dowel rods until there is confidence in positioning and comfort in running the testing system.
-
11
For our testing set-up, we have a program that will load the specimen when a start button is clicked. It will automatically load the specimen at our set rate, while recording the data, and display real-time load-deflection results on the screen. The program has a reset button programmed into it, so that at any point during the test, if pressed, it will automatically bring the testing system back to the starting position.
Test bones
Keep the bone hydrated at all times. If the bone has been out of PBS for more than a few seconds because the test is delayed for whatever reason, return the bone to the petri dish and rehydrate.
-
12
Pull the bone out of the tube and place it in the petri dish.
-
13
Using gauze, remove soft tissue from the bone, if there is any remaining. Put the bone back in the petri dish.
-
14
In the lab notebook, note which bones are being testing, so analysis of the correct bone can be performed later.
-
15
Place the bone in the 4-point bending fixture, with the anterior side facing down, and the distal end of the bone to the left (Fig. 9c). Make sure the bone is centered in the testing fixture from front to back as well.
We prefer testing in the posterior-to-anterior direction, because this subjects the anterior side of the bone to tensile loads. Although the anterior and posterior sides are comprised of lamellar tissue, the organization of the anterior side seems to handle tensile loads better than the posterior side, which results in greater post-yield deflection compared to loading the samples in the anterior-to-posterior direction. The stiffness and maximum load are essentially the same for the two loading directions. -
16
Adjust the loading points, if needed, to ensure that all four points are in contact with the bone.
-
17
Load the bone at a rate of 0.05mm/sec, until the bone breaks. Usually there will be a distinct popping noise. If able to monitor the load and displacement data during the test, there will be a significant drop in the load when the bone has broken. Depending on the mouse being tested, it may be observed that some of the bones will deflect more before breaking. If the bone keeps bending, stop the test when the load reading decreases to less than 50% of the maximum load.
The displacement rate may affect whole bone mechanical properties, particularly post-yield deflection. In general, post-yield deflection is inversely related to the displacement rate. A brittle bone generally shows low post-yield deflection no matter how fast it is loaded. A normal bone will show low post-yield deflection at faster loading rates. If it is suspected that a perturbation may make the bone more brittle, we recommend using the 0.05 mm/sec displacement rate to exaggerate differences between the control and the experimental groups. -
18
Note the location of the break in the lab notebook. Also note any abnormalities that may have occurred during the test or if there were any oddities about the bone tested. Entries could look like “Test1. B6 mouse1 broke in middle”.
-
19
In 4-point bending, the bone can break in the middle, right, or left sides. Because of the design of the testing fixtures, the bone can break anywhere between the two upper points.
-
20
Place the broken bone back in the tube. Place this tube in the pile of already tested bones.
-
21
Repeat with all the bones, until finished.
Data analysis
-
22
Analysis of the load-deflection curves can be done via Excel, or by building a custom analysis program in any number of software packages such as MatLab or LabView (Fig. 10).
Whole bone mechanical properties include measures of stiffness, maximum load, yield load and deflection, post-yield deflection, and work-to-fracture:Stiffness = a measure of how much a bone deforms under a given load. For example, a steel rod is much stiffer (deforms less under load) compared to an aluminum rod.Maximum load = the highest load a bone can sustain prior to fracturing. Note that in whole bone mechanical tests, it is convention to use the term maximum load and not strength, which is often used incorrectly. Strength is similar to maximum load in concept (i.e., greatest load sustained) but this term is used to describe tissue-level mechanical properties, when the effects of geometry have been taken into consideration.Yield point = refers to the point when a loaded structure undergoes permanent changes, such as the accumulation of damage. The structure is now more compliant (less stiff) and this is reflected on load-deflection graphs as a bending in the curve. Because the curves generally show a gentle change in slope, one simple way to assess the yield point in a standardized way is to multiply the stiffness by 0.9 and then find the point on the curve where this degraded stiffness intersects. There is no hard and fast rule for how to define yielding and it will be up to the investigator to standardize this as they see fit.Post-yield deflection = the amount of deflection a structure undergoes after sustaining damage (i.e., yielding) and prior to failing. A brittle material will show very little post-yield deflection prior to failing, whereas a ductile material will show a rather large deflection prior to failing.Work-to-fracture = calculated as the area under the load-deflection graph. This particular mechanical property is often difficult to interpret given that the area under the graph depends on both the height of the curve (i.e., maximum load) and width of the curve (i.e., post-yield deflection). If a mutation is associated with a reduction in work-to-fracture, the first thing you need to do is establish whether there was a change in maximum load and/or a change in post-yield deflection. This is important because the skeletal changes leading to reduced maximum load are very different from those leading to reduced post-yield deflection. -
23Bending stiffness (EI) is an important property that can be calculated. This measure adjusts for the test fixture geometry. The equation for EI takes into account the load, deflection, and the geometry of the testing set-up.
Where, P/y = stiffness from the load-deflection curve, L = span length of the lower two supports, a = span of the upper loading points.
Fig. 10.
Alternate Protocol: WHOLE BONE 3-PT BENDING TESTS
Mechanical testing with a 3-point testing fixture is similar to a 4-point testing fixture. Loading rate and sampling frequency are the same. The biggest difference between the two different methods is where the bone will break. For 3-point bending, the bone will break in the middle, where the single upper loading point comes into contact with the bone. For 4-point bending, the bone can break anywhere between the top two points. Choosing to test with a 3-point fixture system versus a 4-point fixture system is really a matter of personal preference. Part of the decision may come down to the machining capabilities available for designing and building the fixtures. Unpublished data from our lab shows no significant difference between testing with 3-or 4-point bending fixtures. We prefer 4-point bending, to allow the bone to break in the mid-shaft where it is weakest. In 3-point bending, the user decides where the bone will break along the diaphysis.
If testing with 3-point bending fixtures, follow the same procedure as the 4-point bending tests. In steps 5 and 6, instead of adjusting four points to be in contact with the bone, you will adjust three to be in contact with the specimen. Start by making sure the bottom two points are in contact with the bone. Then adjust the top (third) point so that is in contact with the bone as well. Test as per the 4-point bending protocol.
Basic Protocol 5: SPINE COMPRESSION TESTING
For vertebral bodies, we use compression testing because this is consistent with the type of loads expected during habitual loading. Unlike long bones, vertebral bodies contain both cortical and trabecular bone and both tissue types determine the overall strength. Both lumbar and caudal vertebrae work for this testing. The caudal vertebrae are a bit easier to work with, just more difficult to interpret in the context of the human skeleton.
Before you begin this protocol, make sure that you can confidently operate your mechanical testing system. You should be able to run a standard warm-up and change the testing procedure using either the software or the system’s front panel, depending on your system. For this protocol your system will need ±5mm travel distance, 0.05mm/sec testing rate, and a 25Hz sampling frequency. The loading rate can be modified, as needed, to be consistent with prior work.
Materials
Mechanical testing system
Lumbar testing fixtures (Fig. 9d) OR caudal testing fixtures (Fig. 9e)
Pick-ups
Glass petri dish
Absorbent pads
Pencil top erasers or other standard
1X Phosphate Buffered Saline (PBS)
Gauze
Laboratory notebook
Testing preparation
-
1
Pull the tubes with bones out of the freezer. Allow them to thaw for an hour before testing.
Make sure the bones are completely thawed before testing.If the spine has been harvested in sections, disarticulate a single vertebra once the section is thawed. Remove as much soft tissue as possible for easier positioning later. Do not damage the bone sample while removing the soft tissue.If there are any other measurements of the vertebrae that you need (e.g. Computed Tomography), make sure to get these before beginning mechanical testing. -
2
Warm up the mechanical testing system according to the manufacturer’s guidelines.
We run our hydraulic system for 15 minutes, cycling ±5mm. -
3
As the machine is warming up, get the testing area set up.
Place an absorbent pad on a surface near the testing system.On the pad, place a petri dish, bottle of PBS, gauze, and pick-upsFill the petri dish with PBS -
4
Once the machine is warmed up, position the testing fixtures, whether lumbar or caudal. Do as much pre-adjustment as possible.
The testing fixtures should line up vertically so that the vertebra will experience an axial load.Our fixtures have a wire that can be used to center the fixtures before the testing platens are put in. If there is not a similar set-up, be sure to check the alignment by very slowly pushing the fixtures together by hand to ensure they are aligned vertically.
Testing
Test standards
-
5
Determine what standard is desired to test the system and the fixtures before testing bones. We use pencil top erasers. Make sure to choose something that will be easily accessible over the course of the study so that the same type of standard can be used to check the system and setup before testing the bones.
-
6
Position the standard the same way as the bone will be positioned. Make sure that the standard is straight in the vertical direction to ensure an axial load. Move the system actuator so that it holds the standard in place without putting load on it. There can be a fine balance to determining this point. That is why we test this with standards that are easy to replace, not with important bone specimens.
-
7
Run the test with a 25 Hz sampling rate and 0.05 mm/sec displacement rate
-
8
Make sure the system is recording the load, displacement, and time of each test.
-
9
If able, set a limit on the actuator distance. Make sure the limit will allow the bones to be tested, but will prevent the testing platens from making contact and damaging the load cell.
-
10
Run several tests until you are confident the testing set-up is working properly.
Test vertebra
-
11
Use the same test protocol that was used to test the standards.
Keep the vertebra in PBS until ready to test to avoid dehydrating the bone.If for some reason, the bone is set-up and something prevents immediate running of the test, but the vertebra back in the PBS to rehydrate. Dehydration will make a bone brittle, and this would be reflected in reduced post-yield deflection and work-to-fracture. -
12
For lumbar spine testing, place the vertebra so that the peg of the fixture goes into the vertebral foramen, with the spinous process facing the back of the machine. Bring the top platen down slowly. It should touch the vertebra without loading it.
-
13
For caudal spine testing, place the vertebra so that the distal end is resting in the slight indentation of the bottom platen and the proximal end fits into the slight indentation in the top platen. Bring the top platen down very slowly. The platen should touch the vertebra without putting load on it.
-
14
Run the test with a 25 Hz sampling rate and 0.05 mm/sec displacement rate
-
15
Set a limit on the actuator distance. Make sure the limit will allow the bones to be tested, but will prevent the testing platens from making contact.
-
16
Make sure the system is recording the load, displacement, and time of each test.
-
17
Repeat for all bone samples.
Data analysis
-
18
To analyze the spine testing data, plot the load-displacement curve. Plot in N-mm for easiest comparison to the literature.
-
19
Determine the stiffness from the slope of the load-displacement curve.
-
20
Determine the maximum load.
Basic Protocol 6: TISSUE-LEVEL MECHANICAL PROPERTIES
Changes in tissue-level mechanical properties can be measured directly in mouse bone, despite the small size. Most studies estimate tissue-modulus from the whole bone bending tests using engineering beam theory. This method should be used with caution. First, the estimates are generally in the 5–15 GPa range. Mouse bone has a tissue-modulus of 25–35 GPa. Second, tissue-modulus estimated from whole bone bending tests does not correlate well with that measured from direct mechanical testing. Third, only tissue-modulus and tissue-strength can be estimated from whole bone bending tests. Estimating tissue-modulus may be sufficient if the experimental and control groups have very different tissue-level mechanical properties. However, if the tissue-level mechanical properties are similar and if the perturbation is expected to alter tissue-toughness, then we recommend spending the time to measure tissue-level mechanical properties directly.
Materials
Scotch-Weld acrylic adhesive DP-810
Alignment device
Brass pots
CNC milling machine (Modela MDX-20, Roland DGA, Irvine, CA, USA)
Distilled water
Micro scissors
Servo-hydraulic materials testing system (Instron model 8872, Instron, Canton, MA, USA)
High-resolution digital video camera (RH1100, Duncan Tech, Auburn, CA, USA)
Video zoom microscope lens (Edmund Industrial Optics, Barrington, NJ, USA)
16 micron silicon carbide particles (McMaster-Carr, Elmhurst, IL, USA)
Digital-image correlation (IMAQ Vision Builder 6.0, National Instruments Corp., Austin, TX, USA)
Basic fuchsin
Caroplastic (Carolina Biological Supply Co., Burlington, NC, USA)
Low-speed diamond-coated wafering saw (Buehler, Lake Bluff, IL, USA)
Digital Exwave HAD 3CCD Camera (Sony, NY, NY, USA)
Light microscope (Zeiss Axioplan2, Carl Zeiss IMT Corp., Minneapolis, MN, USA)
Procedure
-
1
Clean the sample.
-
2
Embed both metaphyses in brass pots with Scotch-Weld acrylic adhesive DP-810. Use an alignment device to ensure the brass pots are aligned.
-
3
Machine the medial and lateral sides of the potted bone samples to generate an anterior and posterior spicule connecting the proximal and distal halves of the femur. We use an inexpensive automated CNC milling machine. The gauge lengths of the spicules should be ~3 mm long with widths of 280 microns. All machining should be conducted under constant water (PBS) irrigation.
The periosteal and endosteal surfaces of each specimen are not machined in order to minimize microstructural defects from machining. -
4
The samples can be clipped out, under a microscope, and loaded in 4-point bending. Alternatively, the samples can be loaded in tension at 10 microns/sec using a servo-hydraulic materials testing system. Minimize bending loads during the test by precisely aligning all test fixtures using a micrometer stage.
-
5
Deformations can be measured using an imaging system consisting of a high-resolution digital video camera and a video zoom microscope lens. Optical tracking of deformation can be enhanced by “peppering” the sample with 16 micron silicon carbide particles.
-
6
Calculate strain values using digital-image correlation by tracking the movement of the silicon carbide particles
-
7
After the sample is loaded to failure, retrieve the failed ends to measure the cross-sectional area.
-
8
Stain the fracture sample with basic fuchsin.
-
9
Embed the sample in Caroplastic.
-
10
Section the sample transversely (200 micron thick samples) using a low-speed diamond-coated wafering saw.
-
11
Fix the section to acrylic slides and polish to a 1 micron finish.
-
12
Image the sample with a digital Exwave HAD 3CCD Camera and a light microscope.
-
13
Measure cross-sectional areas for 3–6 sections/sample and average the values.
-
14
Divide all force data by the cross-sectional area to calculate stress. Plot stress against the strain determined from the optical tracking system.
-
15
From the stress/strain curves, calculate strength, post-yield strain, work-to-failure, and Young’s modulus. Calculate modulus values from the initial, linear portion of the curve before yield. Yield can be defined in the traditional sense using the 0.2% offset method.
-
16
The overall accuracy of the mechanical testing and imaging methods can be verified using milled aluminum which has a known elastic modulus of 70 GPa.
Basic Protocol 7: ASH WEIGHT, WATER CONTENT, AND BONE DENSITY PROTOCOL
Assessing ash content is a relatively simple first step toward testing if a genetic or environmental perturbation altered matrix composition. This provides a measure of the degree of mineralization. Additional tests using Raman or FTIR imaging can be conducted to generate more refined information about the changes in mineral and collagen. Variation in the degree of mineralization is small, on the order of 2–4%. This variation can result in rather large differences in tissue-level mechanical properties. As such, establishing a consistent method for weighing and ashing is important to obtain meaningful results. We recommend that each lab verify the times listed below so that each weight measure represents the steady state value.
It is important to develop a standard that can be tested over time using this same protocol. This is important to ensure that the system is giving consistent results and no drift is occurring. An aluminum ring standard is a great option because it has known properties (density = 2.7 g/cc) and is easily obtainable.
The following ash weight protocol is only appropriate if the overall specimen weight is greater than 8–10 mg.
Additionally, this protocol is only designed for cortical bone specimens (murine or cadaveric) with very little fat. It is not to be used with specimens that have closed medullary canals. However, it has been validated (Jepsen, unpublished) that there is no difference between defatted and non-defatted densities for mouse bone.
Materials
Distilled water
Vacuum Oven (Fisher Scientific Isotemp or Eurotherm 91e Blue)
Strain gauge wire
Ashing Oven (Thermo Lindberg Blue M)
Delicate task wipes
2mL Eppendorf tubes
Centrifuge (Fisher Scientific accuSpin Micro 17)
Sample preparation and measurements
This is typically a three day procedure for one sample set. First day steps 1–4, second day steps 5 and 6, final day rest of step 6.
-
1
Remove all soft tissue (periosteal tissue, marrow, etc.) under a stereomicroscope. Submerge specimens in distilled water.
-
2
Degas by placing the submerged specimens in 5–7 mmHg vacuum for 2 hours. Remove from vacuum and let stand for an hour at atmospheric conditions.
-
3
Measure submerged weight in water by hanging the specimen from a fine wire and record the weight while the specimen is suspended in distilled water (Fig. 11).
Fig. 11.
Measure hydrated weight in air
-
4
Place a delicate task wipe at the bottom of an Eppendorf tube.
-
5
Place the specimen in the tube; be sure to close the cap to control humidity.
-
6
Centrifuge at 8000g for 10 minutes
-
7
Weigh the specimen
Weigh only 4–5 specimens at a time to reduce errors from further drying out.
Measure dried weight
-
8
Put specimen in 80 deg C oven for at least 3–5 hours. However, overnight is optimal.
-
9
Remove from oven and weigh in air.
Measure ash weight
-
10
Place specimens in 600 deg C oven for 18 hours on ceramic plates or in crucibles.
-
11
Remove from oven and weigh in air.
-
12
Submerge specimen in distilled water.
-
13
Degas (see step 2)
-
14
Measure submerged weights using the fine wire
Calculations
-
15
Volumes
Hydrated Bone Volume = Hydrated weight in air − submerged weightDry Bone Volume = Dry weight in air − submerged weightAsh Volume = Ash weight in air − Submerged ash weight -
16
Densities
Hydrated Bone Density = Hydrated weight in air/Hydrated Bone VolumeDry Bone Density = Dry weight in air/Dry Bone VolumeAsh Density = Ash weight in air/Ash Volume -
17
Contents
Water content = (Hydrated weight in air − Dried weight)/Hydrated weightAsh content = Ash weight/Hydrated weight or Ash weight/Dry weightOrganic + Carbon Dioxide = (Dry weight − Ash weight)/Hydrated weight
Basic Protocol 8: SYSTEMATIC DATA ANALYSIS PROTOCOL
Below is an approach to assess the functional and morphological differences between knock-out (KO) and wild type (WT) mice.
Functional analysis
-
1
Test if the mutation affected bone stiffness.
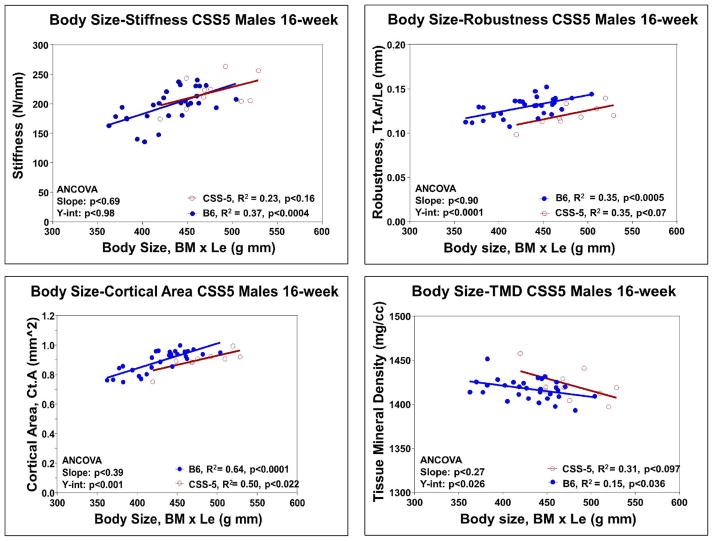
For the KO and WT, regress stiffness against body mass or body mass x length. (Fig. 12a)Test for differences in the slope and y-intercept using ANCOVA. -
2
Test if the mutation affected maximum load.
For the KO and WT, regress maximum load against body mass or body mass x length.Test for differences in the slope and y-intercept using ANCOVA. -
3
Test if the mutation affected post-yield deflection.
For the KO and WT, regress post-yield deflection against body mass or body mass x length.Test for differences in the slope and y-intercept using ANCOVA.PYD does not typically correlate with body mass or body mass x length. -
4
Test if the mutation affected work-to-fracture.
For the KO and WT, regress work-to-fracture against body mass or body mass x length.Test for differences in the slope and y-intercept using ANCOVA.
Fig. 12.
Alternative analysis
Use a general linear model to evaluate the above functional characteristics for each group, using body mass or body mass length as a covariant.
If the KO and WT do not show large differences in body mass, it is acceptable to test for differences in the mechanical properties using a t-test. It is highly advised to first check how each mechanical property varies with body mass to confirm that the KO did not affect the relationship between mechanical properties and body mass that may not be predicted based an analysis of group means alone (e.g., a KO may not show a difference in stiffness compared to WT, but may show a different relationship between stiffness and body mass as determined based on ANCOVA).
Morphological analysis
-
5
Test if the mutation affected periosteal expansion.
For the KO and WT, regress total area (Tt.Ar) against body mass or body mass x length.Test for differences in the slope and y-intercept using ANCOVA. -
6
Test if the mutation affected the relationship between periosteal expansion and growth in bone length.
For the KO and WT, regress robustness (Tt.Ar/Le) against body mass or body mass x length (Fig. 12b).Test for differences in the slope and y-intercept using ANCOVA. -
7
Test if the mutation affected marrow expansion.
For the KO and WT, regress marrow area (Ma.Ar) against body mass or body mass x length.Test for differences in the slope and y-intercept using ANCOVA. -
8
Test if the mutation affected the accrual of bone mass.
For the KO and WT, regress cortical area (Ct.Ar) against body mass or body mass x length (Fig. 12c).Test for differences in the slope and y-intercept using ANCOVA. -
9
Test if the mutation affected the tissue-mineral density or ash content.
For the KO and WT, regress TMD or ash content against body mass or body mass x length (Fig. 12d).Test for differences in the slope and y-intercept using ANCOVA.Typically TMD is not highly correlated with body mass or body mass length. -
10
Alternate analysis
Generate a general linear model with each of the above traits to compare KO and WT, using body mass or body mass length as a covariant. -
11
For vertebral bodies, the cortical shell should be analyzed in the same manner as that described for the femoral diaphysis. To assess how the mutation affected bone volume fraction (BV/TV).
Determine how trabecular thickness and trabecular spacing vary with body mass or body mass x length as factors contributing to stiffness.
Furthermore, if a change in BV/TV is found in a region, also analyze the cortical area around that region for subsequent changes.
NOTE: The variation in BV/TV should be largely explained by Tb.Th and Tb.N. This should be checked to confirm the scanning was conducted properly. Further, this check provides insight into the underlying biological factors contributing to variation in BV/TV. Variation in Tb.N may result from excessive resorption near the growth plate or excessive resorption in the metaphyseal region. Variation in Tb.Th may indicate an imbalance between osteoblastic formation and osteoclastic resorption.
NOTE: Mouse bone is extremely adaptive. If the KO resulted in a more slender phenotype, then this would be associated with reduced Ct.Ar relative to body size and increased TMD. Not seeing these additional changes would likely also be associated with differences in stiffness and maximum load relative to body mass. There are no norms specifying the exact amount of changes in Ct.Ar and TMD expected for a slender phenotype. At this point, it is sufficient to interpret the changes in the context of whole bone mechanical properties. Variation in whole bone mechanical properties is best interpreted in the context of how multiple traits are affected by the mutation. Table 1 presents several scenarios for how a KO may alter structural and material properties and the subsequent effects on whole bone mechanical properties.
Table 1.
Expected changes in functional, morphological and compositional traits
| Robustness | Ct.Ar | TMD | Stiffness | Max Load |
|---|---|---|---|---|
| Slender | Reduced | Increased | No change | No change |
| Slender | Very reduced | No change | Reduced | Reduced |
| No change | Reduced | No change | Reduced | Reduced |
| No change | Increased | Increased | Increased | Increased |
| Increased | Increased | Reduced | No change | No change |
| Increased | Increased | No change | Increased | Increased |
Commentary
Background Information
Most physiological systems are considered complex adaptive systems because they are capable of coordinating multiple traits simultaneously in order to generate organ-level function or homeostasis (Jepsen, 2009; Marder and Goaillard, 2006; Nadeau et al., 2003; Waddington, 1942; Wright, 1921). For the skeletal system, a key function is that bone should be sufficiently stiff and strong to support the loads imposed during habitual loading. Consequently, genetic and environmental perturbations affecting one trait are often accompanied by compensatory changes in other traits (Jepsen et al., 2010; Jepsen et al., 2009; Jepsen et al., 2007). The challenge is to figure out which alterations can be attributed to the perturbation and which result from adaptive changes associated with establishing mechanical function. For example, early research in transgenic mouse models revealed that a mutation affecting type I collagen gene expression led to an age-related increase in bone size (Bonadio et al., 1993). This adaptive response occurred presumably to offset the reduced tissue-stiffness associated with the type I collagen mutation (Jepsen et al., 1997). If the mice were assessed at a single adult age, one may conclude that producing less collagen makes a bigger bone. This statement is not entirely incorrect, but it is certainly misleading particularly in the context of potential treatment strategies for osteoporosis.
A noteworthy example of how a genetic mutation can affect bone morphology but not bone strength was presented by Maloul et al (Maloul et al., 2006). Gdf7 (BMP-12), which is typically thought to regulate soft-tissue healing (Lou et al., 2001), has been shown to also affect proliferation and phenotypic expression of osteoblastic (Ros 17/2.8) cells in culture (Furuya et al., 1999). Interestingly, the Gdf7 deficiency impaired sub-periosteal expansion of femora leading to a slender (narrow) phenotype, but did not affect bone strength. Thus, the skeletal system in the mutant mice altered tissue-level mechanical properties to compensate for the slender phenotype to establish normal strength.
Critical Parameters and Troubleshooting
Basic Protocol 1: Bone harvesting
As mentioned in the introduction to Basic Protocol 1, bone harvesting is straightforward as long as a one possesses the prerequisite knowledge of anatomy and exercises particular care to avoid shearing the epiphysis.
Support Protocol: MMA Solution Protocol
When making this solution, several safety guidelines bear repeating. All procedures must be performed under an approved chemical fume hood. Never use metal or other conductive instruments to weigh out benzoyl peroxide.
DO NOT open any MMA bottle until thawed and warmed to room temperature. It is important to avoid introducing water into the solution, as it can mess up the embedding process.
If at any point the reaction proceeds too quickly, the MMA bottle can be placed in −20°C to cool it off and pause the polymerization process.
Basic Protocol 2: Plastic Embedding Protocol
Keep in mind that this protocol specifically details plastic embedding, but does not discuss labeling of bones beforehand for epifluorescent microscopy. Please refer to the second paragraph of the introduction for a brief overview of the labeling procedure we use, noting particularly that injection timing varies depending on the age of the mouse.
During the embedding process, exercise extreme caution to avoid introducing water into the sample(s) after washing samples during the dehydration procedure; particularly, avoid condensation of the MMA II solution by allowing it to warm to room temperature prior to opening. Because all four MMA solutions are used during Basic Protocol 2, all precautions mentioned for Support Protocol 1 (MMA Solution Protocol) apply here as well.
Basic Protocol 3: Computed Tomography Protocol
For nanoCT imaging, as with all CT procedures, material selection and purity are critical. Most steps within Basic Protocol 3 require empirical testing to find optimal settings to obtain the highest signal-to-noise ratio possible. The exact procedure we outline is tailored for our scanning system and software, but the general concepts should be generally applicable.
Bone bending tests (Basic Protocols 4 and 5 and Alternate Protocol 1)
Bending tests are destructive, so utilize samples for other (non-destructive) assays prior to the bending tests. Make sure you are thoroughly familiar with your selected testing machinery, follow strict sample organization/labeling procedures, and maintain sample hydration. Make liberal use of practice dowels to ensure that machinery parameters are appropriate and data recording is on-line.
Basic Protocol 7: Ash Weight, Water Content, and Bone Density
Again, note the importance of developing and validating a standard that can be tested over time (as mentioned in the introduction of Basic Protocol 7, we suggest an aluminum ring). Additionally, the specific parameters for this protocol should (based on unpublished observations) apply for both defatted and non-defatted mouse bone, but published results (to date) have been demonstrated only for the former.
Also, we reiterate that the ash weight protocol presented here is only appropriate if the overall specimen weight is greater than 8–10 mg.
Anticipated Results
The protocols in this manuscript are meant to provide a first-line phenotypic view of bone morphology and biomechnical characteristics to drive hypotheses and subsequent experiments for mechanistic investigations. As such, we try to present individual protocols to be accessible to researchers without extensive bioengineering backgrounds, but acknowledge that a certain level of familiarity in working with mouse bone, operating specialized machinery, and drawing appropriate conclusions for in-depth investigations is required.
Ignoring technical expertise with specialized equipment, the fundamental steps for bone harvesting, mounting, and imaging are relatively straightforward provided care is taken to follow the provided instructions. Please note, however, that these protocols do not discuss experimental design, and only touch on data interpretation. For additional insight into the many considerations required for detailed mouse bone experiments, refer to the references throughout this manuscript.
Time Considerations
It is challenging to assign time frames for many of these protocols owing to the anticipated variance in experimenter experience, access to automated machinery, and familiarity/expertise with operating and maintaining advanced equipment. Assuming a moderate level of experience, bone harvesting is a relatively quick process, taking approximately 5–10 minutes per mouse to harvest long bones and spine. Making MMA solutions requires several days, although most of this comprises waiting time. Embedding bones is a time-consuming process due to wait times, and several weeks should be allotted from beginning to end. Whole bone mechanical testing is a relatively quick procedure, taking approximately 5 minutes to mechanically test a femur or spine. Tissue-level mechanical testing, however, is extremely time consuming and requires tremendous attention to detail. This type of experiment would need approximately 8 hours of work for each bone sample. For ash content analysis, the individual weighing procedures are not time consuming (1–2 minutes per sample). However, the overall time allotted to conduct these studies is 3–5 days given the wait times for drying and ashing. Morphological analyses using the nanoCT is actually quite straightforward and we estimate it takes 2–3 hours to scan a bone, 10–15 minutes to reconstruct the 3D image, 1 hour for calibration and image processing, and 10–20 minutes to analyze depending on whether it is a femur or spine being studied.
References
- Bonadio J, Jepsen KJ, Mansoura MK, Jaenisch R, Kuhn JL, Goldstein SA. A murine skeletal adaptation that significantly increases cortical bone mechanical properties. Implications for human skeletal fragility. J Clin Invest. 1993;92:1697–1705. doi: 10.1172/JCI116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya K, Nifuji A, Rosen V, Noda M. Effects of GDF7/BMP12 on proliferation and alkaline phosphatase expression in rat osteoblastic osteosarcoma ROS 17/2.8 cells. J Cell Biochem. 1999;72:177–180. doi: 10.1002/(sici)1097-4644(19990201)72:2<177::aid-jcb2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ. Systems analysis of bone. Wiley Interdiscip Rev Syst Biol Med. 2009;1:73–88. doi: 10.1002/wsbm.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Courtland HW, Nadeau JH. Genetically-determined phenotype covariation networks control bone strength. J Bone Miner Res. 2010;25:1581–1593. doi: 10.1002/jbmr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Cordova M, Nadeau JH. Phenotypic integration of skeletal traits during growth buffers genetic variants affecting the slenderness of femora in inbred mouse strains. Mamm Genome. 2009;20:21–33. doi: 10.1007/s00335-008-9158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Terranova CJ, Nadeau JH. Genetic randomization reveals functional relationships among morphologic and tissue-quality traits that contribute to bone strength and fragility. Mamm Genome. 2007;18:492–507. doi: 10.1007/s00335-007-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Schaffler MB, Kuhn JL, Goulet RW, Bonadio J, Goldstein SA. Type I collagen mutation alters the strength and fatigue behavior of Mov13 cortical tissue. J Biomech. 1997;30:1141–1147. doi: 10.1016/s0021-9290(97)00088-2. [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Maloul A, Rossmeier K, Mikic B, Pogue V, Battaglia T. Geometric and material contributions to whole bone structural behavior in GDF-7-deficient mice. Connect Tissue Res. 2006;47:157–162. doi: 10.1080/03008200600719142. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Nadeau JH, Burrage LC, Restivo J, Pao YH, Churchill G, Hoit BD. Pleiotropy, homeostasis, and functional networks based on assays of cardiovascular traits in genetically randomized populations. Genome Res. 2003;13:2082–2091. doi: 10.1101/gr.1186603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;14:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wright S. Correlation and causation. Journal of Agricultural Research. 1921;20:557–585. [Google Scholar]