Abstract
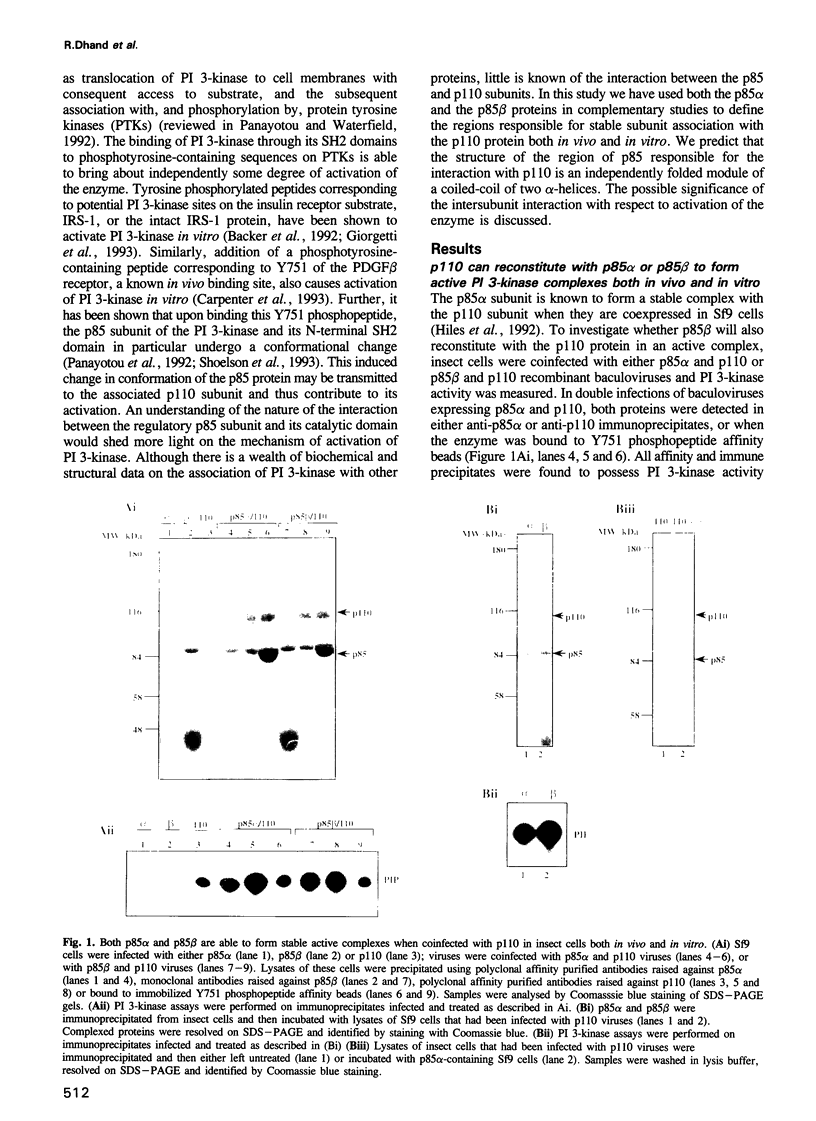
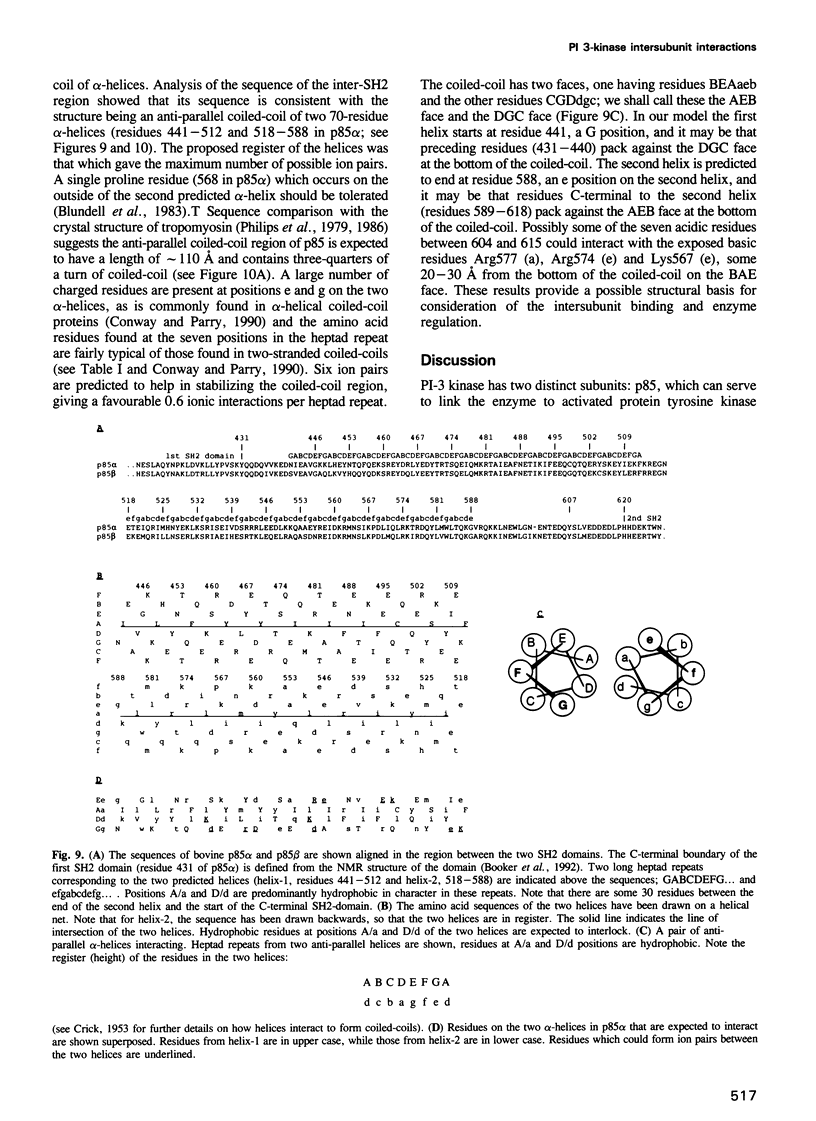
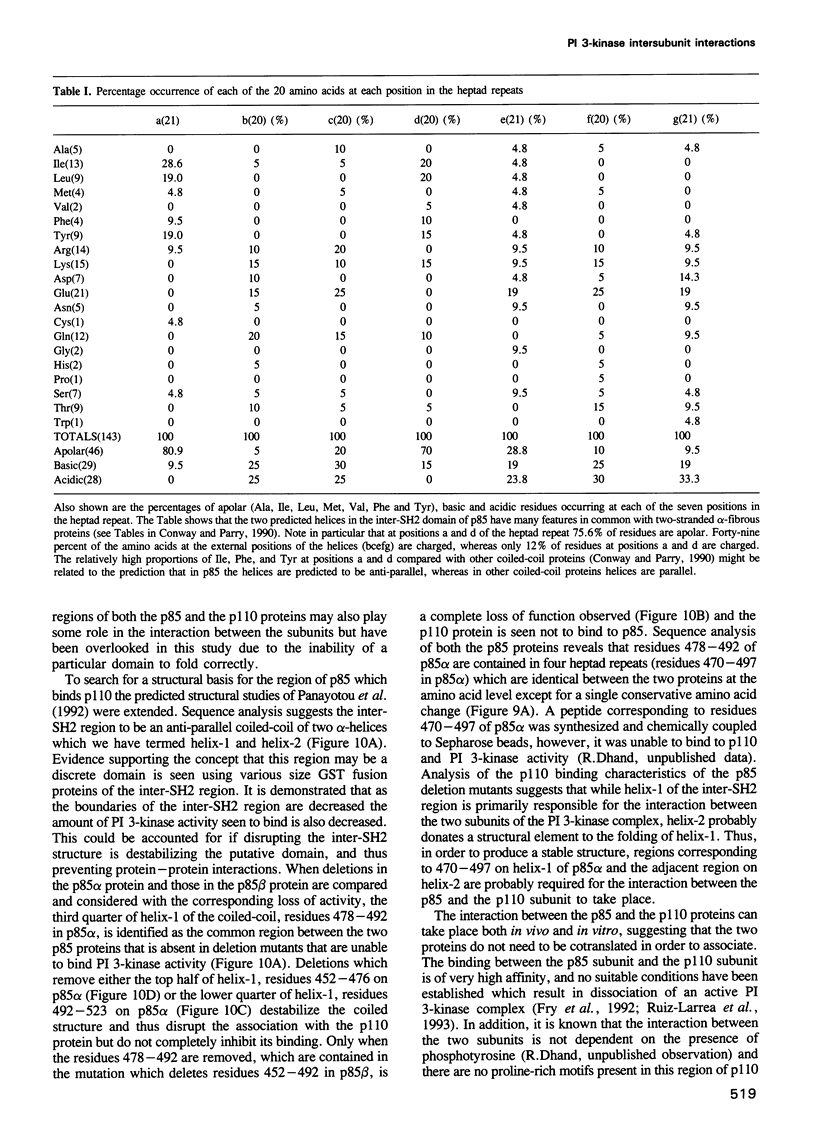
Phosphatidylinositol (PI) 3-kinase has an 85 kDa subunit (p85 alpha) which mediates its association with activated protein tyrosine kinase receptors through SH2 domains, and an 110 kDa subunit (p110) which has intrinsic catalytic activity. Here p85 alpha and a related protein p85 beta are shown to form stable complexes with recombinant p110 in vivo and in vitro. Using a panel of glutathione S-transferase (GST) fusion proteins of the inter-SH2 region of p85, 104 amino acids were found to bind directly the p110 protein, while deletion mutants within this region further defined the binding site to a sequence of 35 amino acids. Transient expression of the mutant p85 alpha protein in mouse L cells showed it was unable to bind PI 3-kinase activity in vivo. Mapping of the complementary site of interaction on the p110 protein defined 88 amino acids in the N-terminal region of p110 which mediate the binding of this subunit to either the p85 alpha or the p85 beta proteins. The inter-SH2 region of p85 is predicted to be an independently folded module of a coiled-coil of two long anti-parallel alpha-helices. The predicted structure of p85 suggests a basis for the intersubunit interaction and the relevance of this interaction with respect to the regulation of the PI 3-kinase complex is discussed.
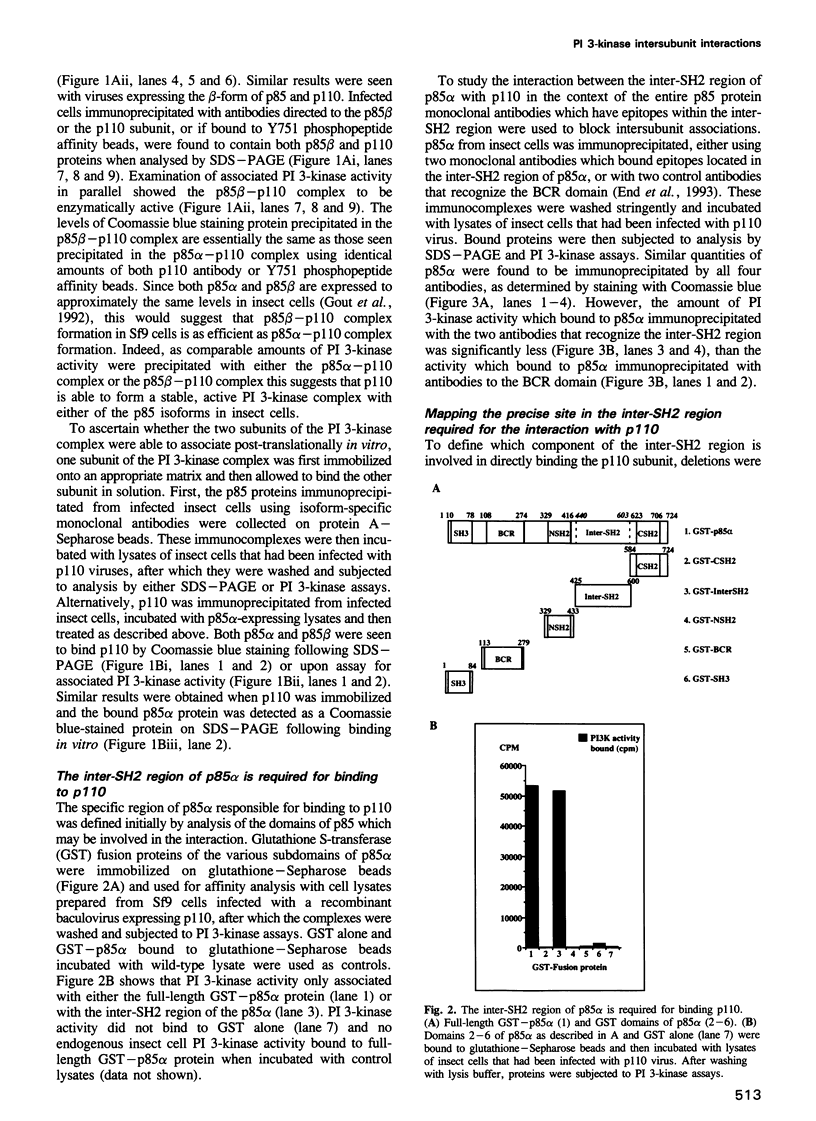
Full text
PDF
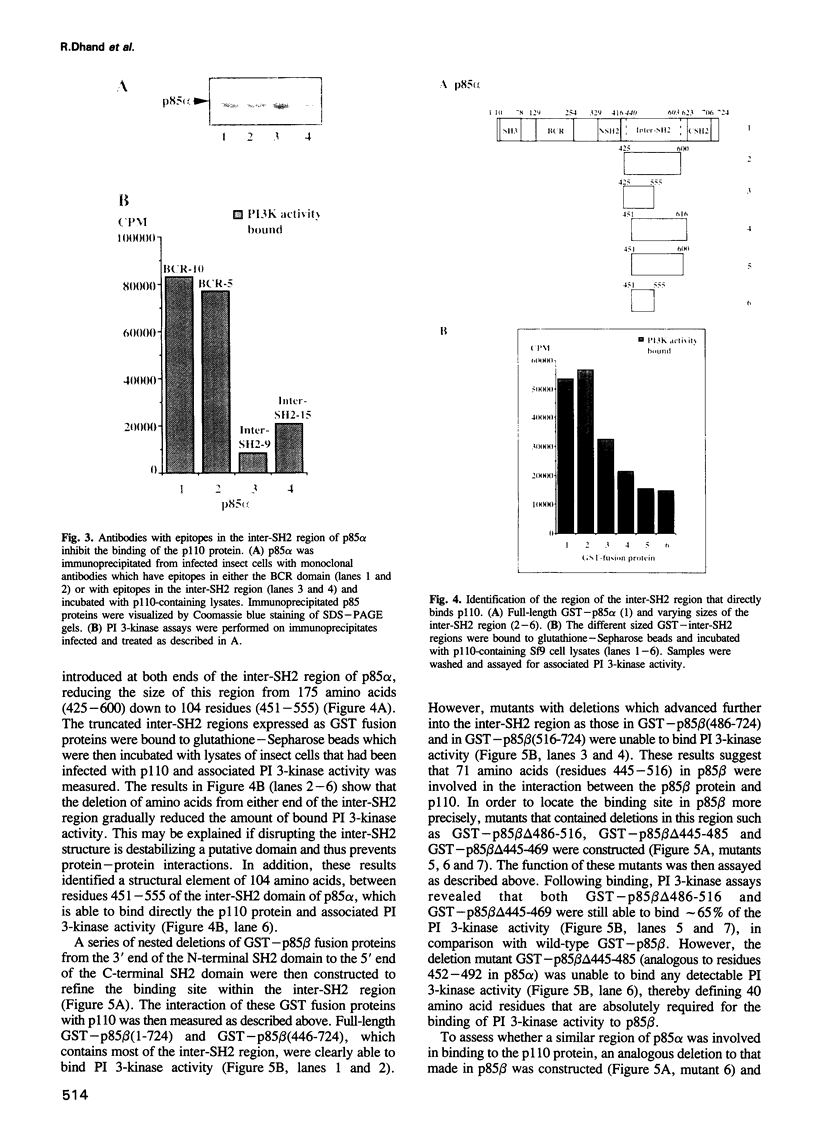



Images in this article
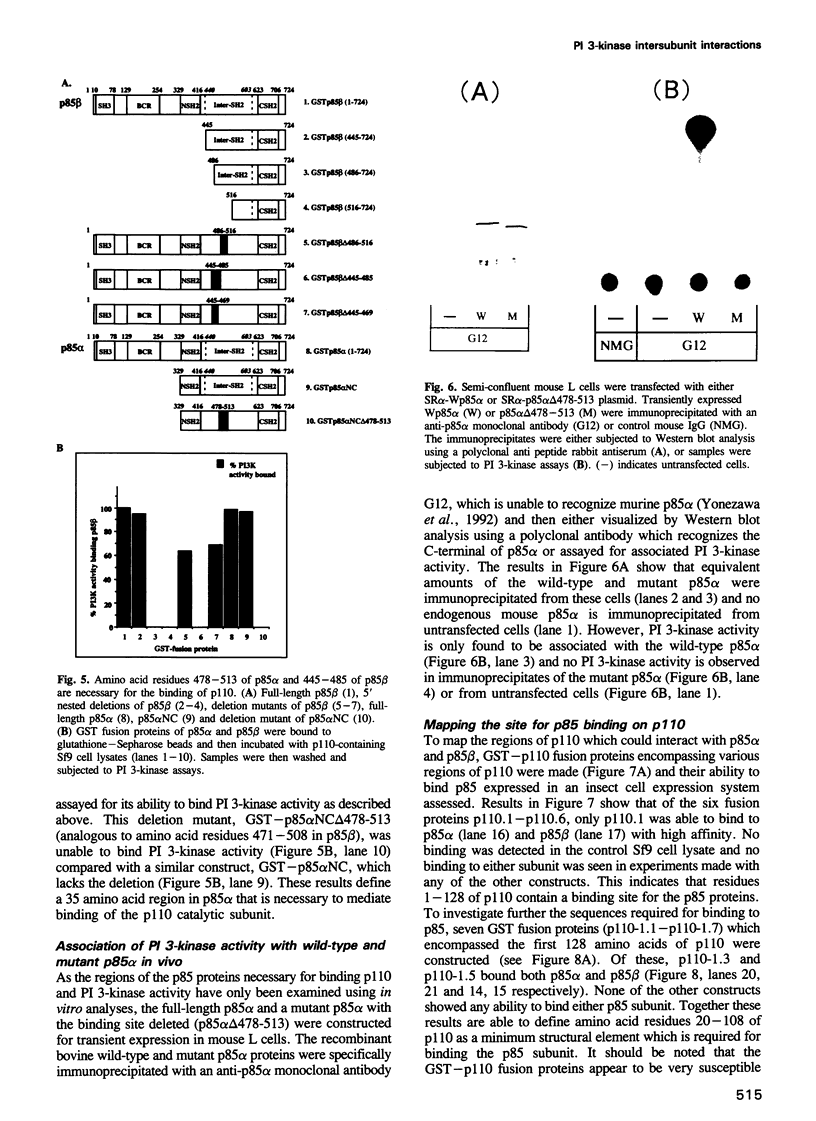
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
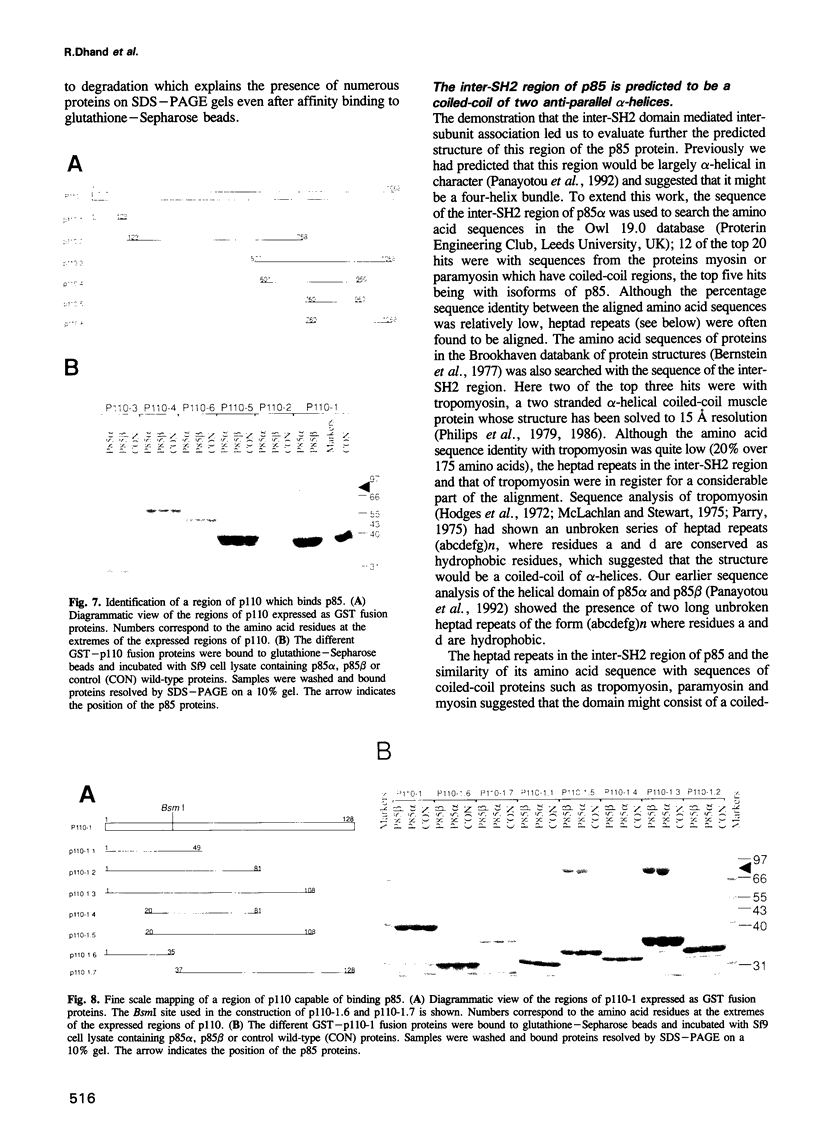
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blundell T., Barlow D., Borkakoti N., Thornton J. Solvent-induced distortions and the curvature of alpha-helices. Nature. 1983 Nov 17;306(5940):281–283. doi: 10.1038/306281a0. [DOI] [PubMed] [Google Scholar]
- Booker G. W., Breeze A. L., Downing A. K., Panayotou G., Gout I., Waterfield M. D., Campbell I. D. Structure of an SH2 domain of the p85 alpha subunit of phosphatidylinositol-3-OH kinase. Nature. 1992 Aug 20;358(6388):684–687. doi: 10.1038/358684a0. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Auger K. R., Chanudhuri M., Yoakim M., Schaffhausen B., Shoelson S., Cantley L. C. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993 May 5;268(13):9478–9483. [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Conway J. F., Parry D. A. Structural features in the heptad substructure and longer range repeats of two-stranded alpha-fibrous proteins. Int J Biol Macromol. 1990 Oct;12(5):328–334. doi: 10.1016/0141-8130(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Kashishian A. In vivo binding properties of SH2 domains from GTPase-activating protein and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993 Mar;13(3):1737–1745. doi: 10.1128/mcb.13.3.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- End P., Gout I., Fry M. J., Panayotou G., Dhand R., Yonezawa K., Kasuga M., Waterfield M. D. A biosensor approach to probe the structure and function of the p85 alpha subunit of the phosphatidylinositol 3-kinase complex. J Biol Chem. 1993 May 15;268(14):10066–10075. [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Martin G. A., Turck C. W., del Rosario M., McCormick F., Williams L. T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992 May 1;69(3):413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- Fry M. J. Defining a new GAP family. Curr Biol. 1992 Feb;2(2):78–80. doi: 10.1016/0960-9822(92)90207-q. [DOI] [PubMed] [Google Scholar]
- Fry M. J., Panayotou G., Dhand R., Ruiz-Larrea F., Gout I., Nguyen O., Courtneidge S. A., Waterfield M. D. Purification and characterization of a phosphatidylinositol 3-kinase complex from bovine brain by using phosphopeptide affinity columns. Biochem J. 1992 Dec 1;288(Pt 2):383–393. doi: 10.1042/bj2880383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Gout I., Dhand R., Panayotou G., Fry M. J., Hiles I., Otsu M., Waterfield M. D. Expression and characterization of the p85 subunit of the phosphatidylinositol 3-kinase complex and a related p85 beta protein by using the baculovirus expression system. Biochem J. 1992 Dec 1;288(Pt 2):395–405. doi: 10.1042/bj2880395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Jackson T. R., Stephens L. R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992 Jul 9;358(6382):157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Lamb J., Allen P. G., Matsudaira P. T. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem. 1992 Jun 15;267(17):11818–11823. [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Ling L. E., Druker B. J., Cantley L. C., Roberts T. M. Transformation-defective mutants of polyomavirus middle T antigen associate with phosphatidylinositol 3-kinase (PI 3-kinase) but are unable to maintain wild-type levels of PI 3-kinase products in intact cells. J Virol. 1992 Mar;66(3):1702–1708. doi: 10.1128/jvi.66.3.1702-1708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975 Oct 25;98(2):293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- Morgan S. J., Smith A. D., Parker P. J. Purification and characterization of bovine brain type I phosphatidylinositol kinase. Eur J Biochem. 1990 Aug 17;191(3):761–767. doi: 10.1111/j.1432-1033.1990.tb19185.x. [DOI] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Panayotou G., Bax B., Gout I., Federwisch M., Wroblowski B., Dhand R., Fry M. J., Blundell T. L., Wollmer A., Waterfield M. D. Interaction of the p85 subunit of PI 3-kinase and its N-terminal SH2 domain with a PDGF receptor phosphorylation site: structural features and analysis of conformational changes. EMBO J. 1992 Dec;11(12):4261–4272. doi: 10.1002/j.1460-2075.1992.tb05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotou G., Waterfield M. D. Phosphatidyl-inositol 3-kinase: a key enzyme in diverse signalling processes. Trends Cell Biol. 1992 Dec;2(12):358–360. doi: 10.1016/0962-8924(92)90042-l. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Waterfield M. D. Phosphatidylinositol 3-kinase: a novel effector. Cell Growth Differ. 1992 Oct;3(10):747–752. [PubMed] [Google Scholar]
- Parry D. A. Analysis of the primary sequence of alpha-tropomyosin from rabbit skeletal muscle. J Mol Biol. 1975 Nov 5;98(3):519–535. doi: 10.1016/s0022-2836(75)80084-2. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986 Nov 5;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Lattman E. E., Cummins P., Lee K. Y., Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979 Mar 29;278(5703):413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]
- Reif K., Gout I., Waterfield M. D., Cantrell D. A. Divergent regulation of phosphatidylinositol 3-kinase P85 alpha and P85 beta isoforms upon T cell activation. J Biol Chem. 1993 May 25;268(15):10780–10788. [PubMed] [Google Scholar]
- Ruiz-Larrea F., Vicendo P., Yaish P., End P., Panayotou G., Fry M. J., Morgan S. J., Thompson A., Parker P. J., Waterfield M. D. Characterization of the bovine brain cytosolic phosphatidylinositol 3-kinase complex. Biochem J. 1993 Mar 1;290(Pt 2):609–616. doi: 10.1042/bj2900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., Homma Y., Takenawa T. Two types of phosphatidylinositol 3-kinase from bovine thymus. Monomer and heterodimer form. J Biol Chem. 1991 May 5;266(13):8108–8114. [PubMed] [Google Scholar]
- Shoelson S. E., Sivaraja M., Williams K. P., Hu P., Schlessinger J., Weiss M. A. Specific phosphopeptide binding regulates a conformational change in the PI 3-kinase SH2 domain associated with enzyme activation. EMBO J. 1993 Feb;12(2):795–802. doi: 10.1002/j.1460-2075.1993.tb05714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hughes K. T., Irvine R. F. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valius M., Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell. 1993 Apr 23;73(2):321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- Volinia S., Patracchini P., Otsu M., Hiles I., Gout I., Calzolari E., Bernardi F., Rooke L., Waterfield M. D. Chromosomal localization of human p85 alpha, a subunit of phosphatidylinositol 3-kinase, and its homologue p85 beta. Oncogene. 1992 Apr;7(4):789–793. [PubMed] [Google Scholar]
- Yonezawa K., Ueda H., Hara K., Nishida K., Ando A., Chavanieu A., Matsuba H., Shii K., Yokono K., Fukui Y. Insulin-dependent formation of a complex containing an 85-kDa subunit of phosphatidylinositol 3-kinase and tyrosine-phosphorylated insulin receptor substrate 1. J Biol Chem. 1992 Dec 25;267(36):25958–25965. [PubMed] [Google Scholar]
- Yu F. X., Sun H. Q., Janmey P. A., Yin H. L. Identification of a polyphosphoinositide-binding sequence in an actin monomer-binding domain of gelsolin. J Biol Chem. 1992 Jul 25;267(21):14616–14621. [PubMed] [Google Scholar]