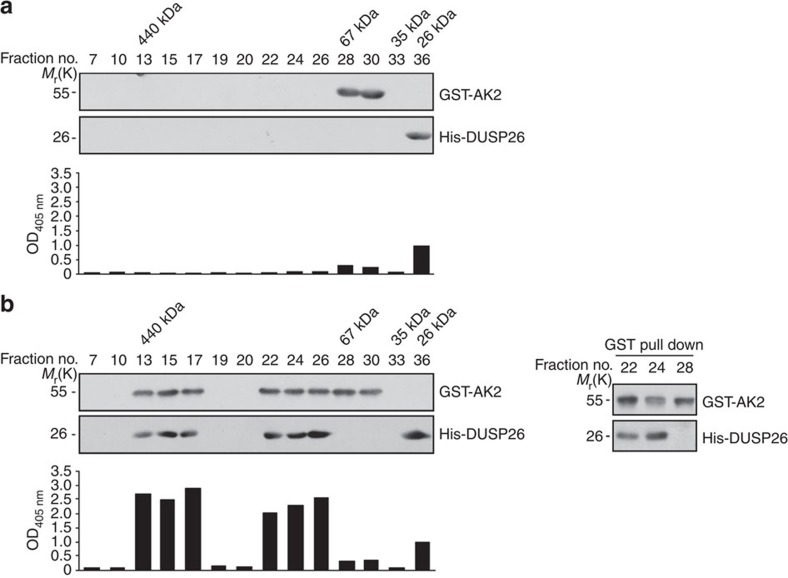
Figure 4. Purified AK2 protein forms a complex with DUSP26 on gel filtration.
(a) Elusion profile and phosphatase activity of each AK2 or DUSP26 protein on size-exclusion chromatography. Purified GST-AK2 or His-DUSP26 protein (each 10 μg) was separated on a Superdex 200 gel filtration column (0.6-ml fractions). Proteins of each fraction were then separated by SDS–PAGE and analysed by western blotting with anti-AK2 or anti-DUSP26 antibody. Each fraction was also assayed for the phosphatase activity using pNPP. (b) The formation of AK2/DUSP26 binary protein complexes on size-exclusion chromatography and stimulation of DUSP26 phosphatase activity by AK2. Purified GST-AK2 and His-DUSP26 proteins (each 10 μg) were combined together for 1 h and then subjected to size-exclusion chromatography as in a. Each fraction was examined by western blotting and phosphatase assays. Protein fractions (number 22, 24 and 28) were subjected to GST pull-down assays and the pulled samples were analysed by western blotting with anti-AK2 and anti-DUSP26 antibodies.