Abstract
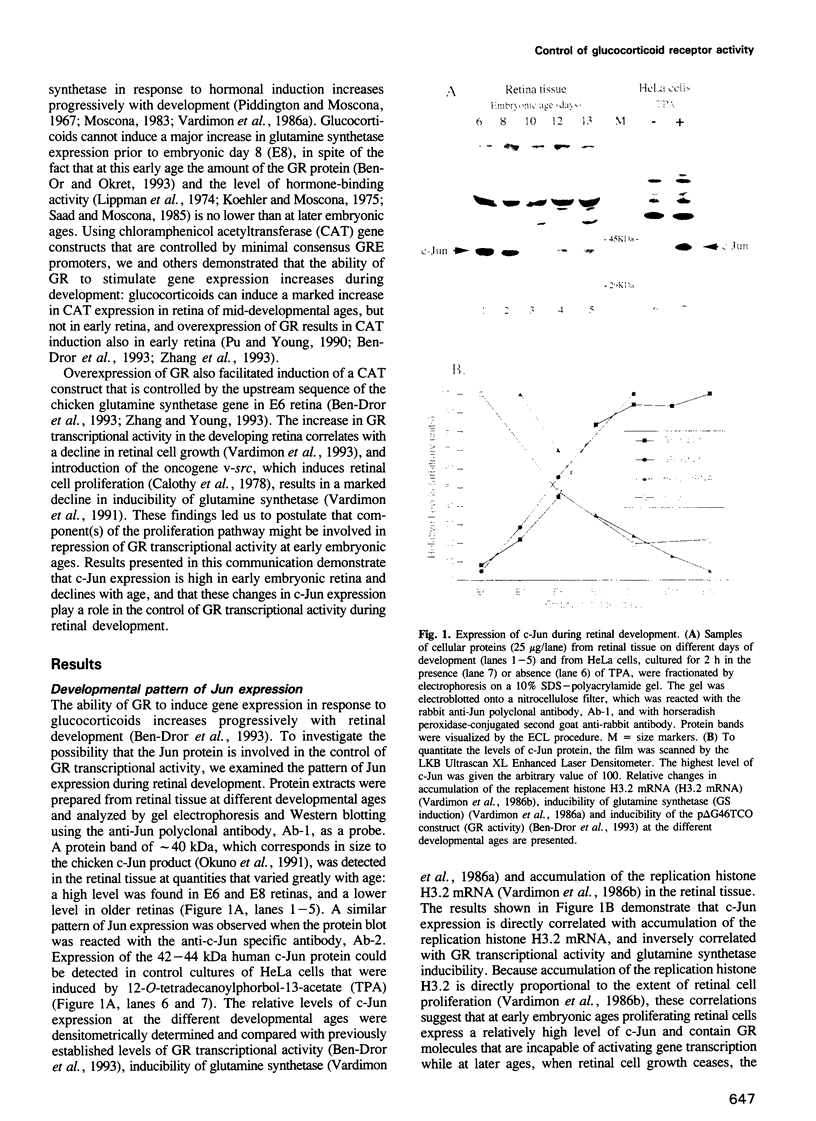
The ability of the glucocorticoid receptor (GR) to induce gene expression in embryonic chicken retinal tissue increases dramatically during development, although the quantity of the receptor molecules does not change greatly with age. This study examines the possible involvement of c-Jun in the developmental control of GR activity. Expression of c-Jun in retinal tissue was high at early embryonic ages and declined during development. Elevation of c-Jun expression in retina of mid-developmental ages by treatment with 12-O-tetradecanoyl-phorbol-13-acetate (TPA), or by introduction of a c-Jun expression vector, caused a pronounced decline in the inducibility of the endogenous glutamine synthetase gene and the transiently transfected CAT constructs p delta G46TCO and pGS2.1CAT, that are controlled by a minimal consensus glucocorticoid response element (GRE) promoter and the glutamine synthetase promoter, respectively. The effect of c-Jun was dose dependent and could be reversed by overexpression of GR. C-Jun-evoked repression of GR activity could be relieved by overexpression of Jun D. Overexpression of Jun D could also elevate the responsiveness of early embryonic retina to glucocorticoids and cause a 5-fold increase in p delta G46TCO induction. The effect of Jun D could be reversed by overexpression of c-Jun. Expression of c-Jun might therefore be important for repression of GR activity at early embryonic ages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., van der Valk M. A., Schönenberger C. A., Flückiger F., LeMeur M., Gerlinger P., Groner B. Ha-ras and c-myc oncogene expression interferes with morphological and functional differentiation of mammary epithelial cells in single and double transgenic mice. Genes Dev. 1988 Nov;2(11):1486–1495. doi: 10.1101/gad.2.11.1486. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Forsham P. H. Tissue effects of glucocorticoids. Am J Med. 1972 Nov;53(5):573–589. doi: 10.1016/0002-9343(72)90154-4. [DOI] [PubMed] [Google Scholar]
- Ben-Dror I., Havazelet N., Vardimon L. Developmental control of glucocorticoid receptor transcriptional activity in embryonic retina. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1117–1121. doi: 10.1073/pnas.90.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Or S., Okret S. Involvement of a C/EBP-like protein in the acquisition of responsiveness to glucocorticoid hormones during chick neural retina development. Mol Cell Biol. 1993 Jan;13(1):331–340. doi: 10.1128/mcb.13.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T. J., Monteclaro F. S., Mitsunobu F., Ball A. R., Jr, Chang C. H., Nishimura T., Vogt P. K. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modification in coding and noncoding sequences. Genes Dev. 1990 Oct;4(10):1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Pessac B. A transformation defective mutant of Rous sarcoma virus inducing chick embryo neuroretinal cell proliferation. Virology. 1978 Aug;89(1):75–84. doi: 10.1016/0042-6822(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Dangy J. P., Mechta F., Hirai S., Yaniv M., Samarut J., Lassailly A., Brun G. Overexpression of avian or mouse c-jun in primary chick embryo fibroblasts confers a partially transformed phenotype. Oncogene. 1990 Oct;5(10):1541–1547. [PubMed] [Google Scholar]
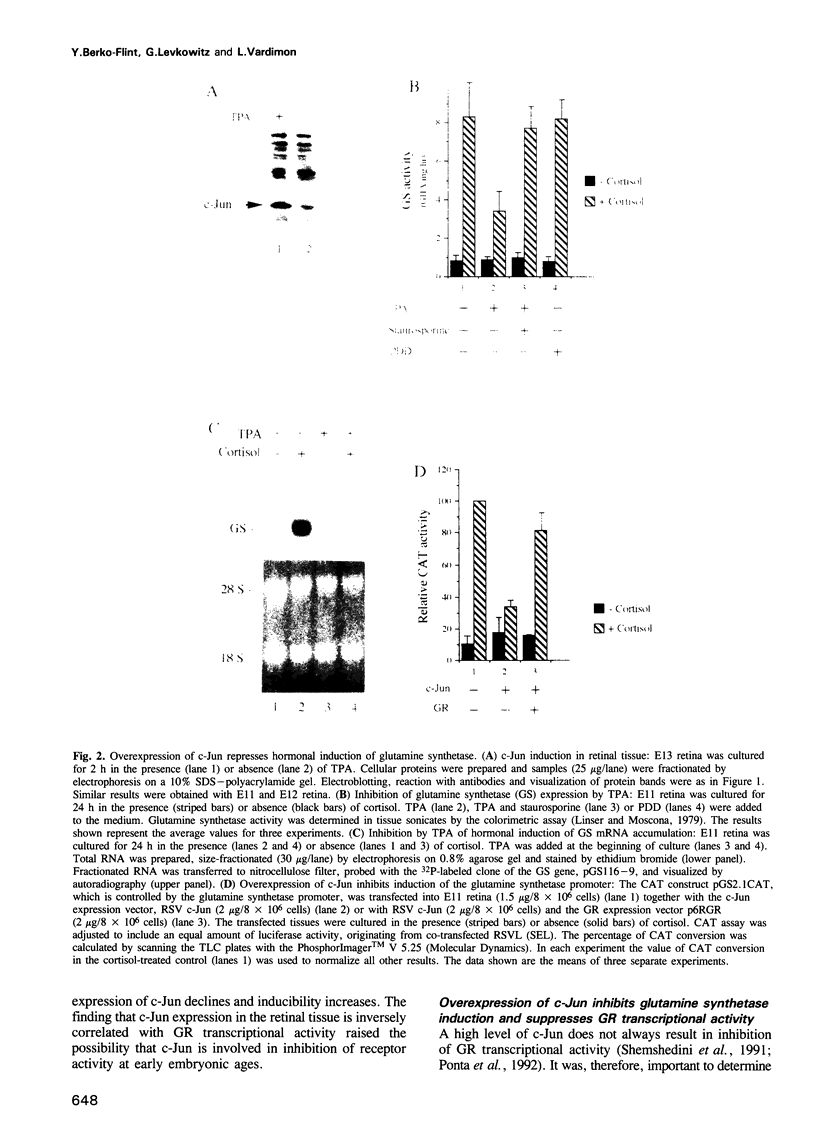
- Castellazzi M., Dangy J. P., Mechta F., Hirai S., Yaniv M., Samarut J., Lassailly A., Brun G. Overexpression of avian or mouse c-jun in primary chick embryo fibroblasts confers a partially transformed phenotype. Oncogene. 1990 Oct;5(10):1541–1547. [PubMed] [Google Scholar]
- Castellazzi M., Spyrou G., La Vista N., Dangy J. P., Piu F., Yaniv M., Brun G. Overexpression of c-jun, junB, or junD affects cell growth differently. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8890–8894. doi: 10.1073/pnas.88.20.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Crook R. B., Louie M., Deuel T. F., Tomkins G. M. Regulation of glutamine synthetase by dexamethasone in hepatoma tissue culture cells. J Biol Chem. 1978 Sep 10;253(17):6125–6131. [PubMed] [Google Scholar]
- Deng T., Karin M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993 Mar;7(3):479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucas V., Spyrou G., Yaniv M. Unregulated expression of c-Jun or c-Fos proteins but not Jun D inhibits oestrogen receptor activity in human breast cancer derived cells. EMBO J. 1991 Aug;10(8):2237–2245. doi: 10.1002/j.1460-2075.1991.tb07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin M., LaRue H., Bernier D., Wrange O., Chevrette M., Gingras M. C., Bélanger L. Enhancer and promoter elements directing activation and glucocorticoid repression of the alpha 1-fetoprotein gene in hepatocytes. Mol Cell Biol. 1988 Apr;8(4):1398–1407. doi: 10.1128/mcb.8.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M., Hutchins J. T., Vogt P. K. The chicken junD gene and its product. Oncogene. 1991 Sep;6(9):1623–1631. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
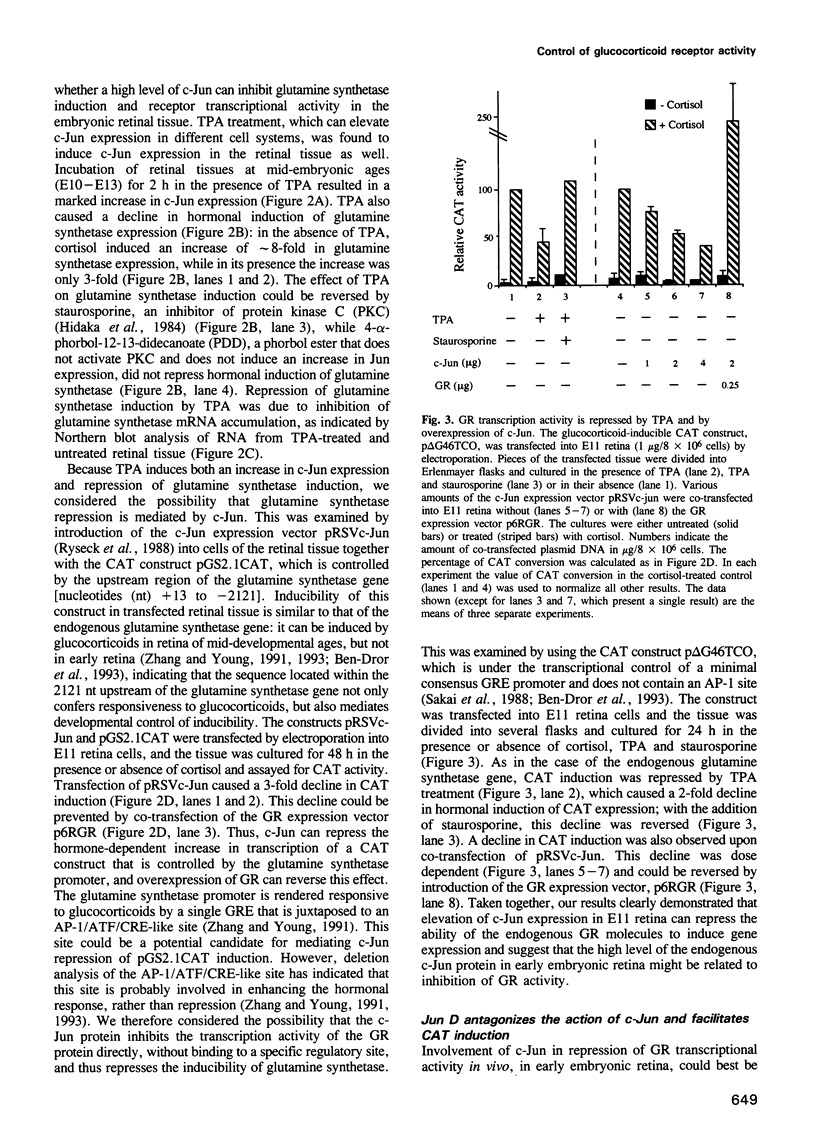
- Hirai S. I., Ryseck R. P., Mechta F., Bravo R., Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989 May;8(5):1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kobierski L. A., Chu H. M., Tan Y., Comb M. J. cAMP-dependent regulation of proenkephalin by JunD and JunB: positive and negative effects of AP-1 proteins. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10222–10226. doi: 10.1073/pnas.88.22.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Moscona A. A. Corticosteroid receptors in the neural retina and other tissues of the chick embryo. Arch Biochem Biophys. 1975 Sep;170(1):102–113. doi: 10.1016/0003-9861(75)90101-0. [DOI] [PubMed] [Google Scholar]
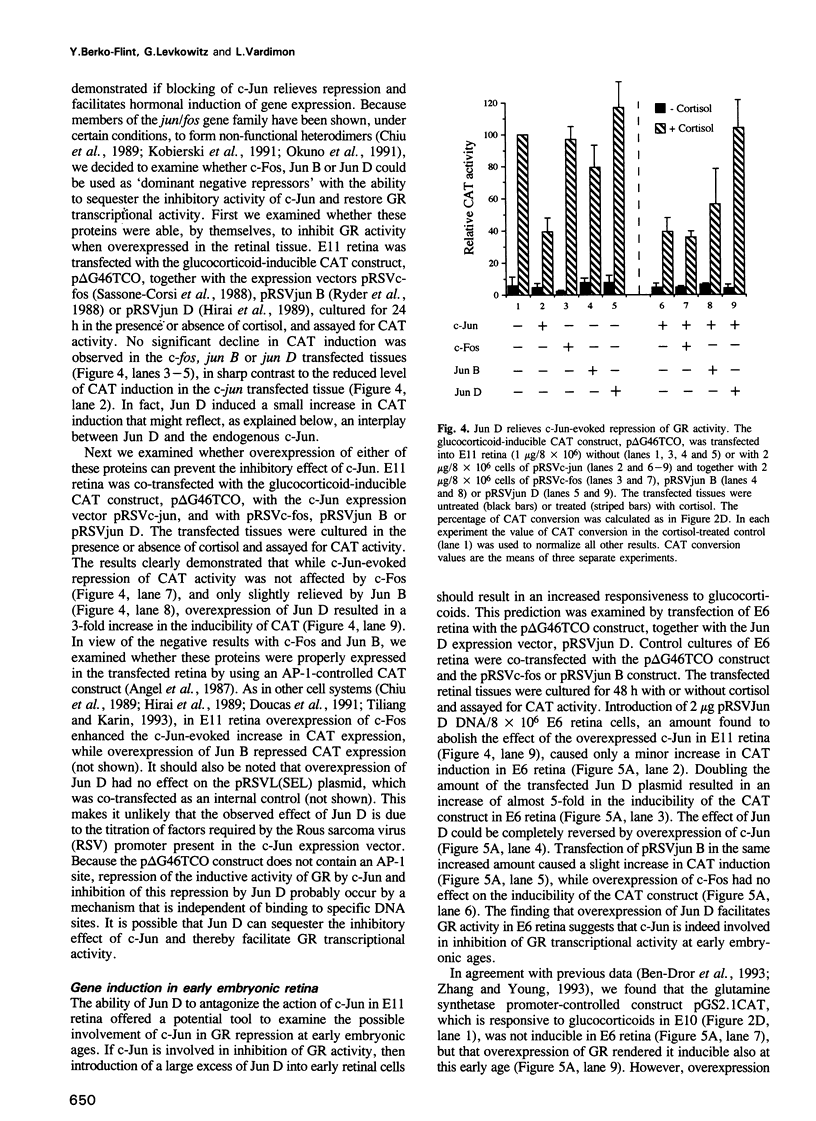
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers J. P., Spelsberg T. C. New concepts in steroid hormone action: transcription factors, proto-oncogenes, and the cascade model for steroid regulation of gene expression. Crit Rev Eukaryot Gene Expr. 1992;2(1):19–63. [PubMed] [Google Scholar]
- Li L., Hu J. S., Olson E. N. Different members of the jun proto-oncogene family exhibit distinct patterns of expression in response to type beta transforming growth factor. J Biol Chem. 1990 Jan 25;265(3):1556–1562. [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman M. E., Wiggert B. O., Chader G. J., Thompson E. B. Glucocorticoid receptors. Characteristics, specificity, and ontogenesis in embryonic chick neural retina. J Biol Chem. 1974 Sep 25;249(18):5916–5917. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordacq J. C., Linzer D. I. Co-localization of elements required for phorbol ester stimulation and glucocorticoid repression of proliferin gene expression. Genes Dev. 1989 Jun;3(6):760–769. doi: 10.1101/gad.3.6.760. [DOI] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J Cell Biol. 1988 Jul;107(1):279–286. doi: 10.1083/jcb.107.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H., Suzuki T., Yoshida T., Hashimoto Y., Curran T., Iba H. Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene. 1991 Sep;6(9):1491–1497. [PubMed] [Google Scholar]
- Piddington R., Moscona A. A. Precocious induction of retinal glutamine synthetase by hydrocortisone in the embryo and in culture. Age-dependent differences in tissue response. Biochim Biophys Acta. 1967 Jul 25;141(2):429–432. doi: 10.1016/0304-4165(67)90120-1. [DOI] [PubMed] [Google Scholar]
- Ponta H., Cato A. C., Herrlich P. Interference of pathway specific transcription factors. Biochim Biophys Acta. 1992 Feb 11;1129(3):255–261. doi: 10.1016/0167-4781(92)90501-p. [DOI] [PubMed] [Google Scholar]
- Pu H. F., Young A. P. Glucocorticoid-inducible expression of a glutamine synthetase-CAT-encoding fusion plasmid after transfection of intact chicken retinal explant cultures. Gene. 1990 May 14;89(2):259–263. doi: 10.1016/0378-1119(90)90014-i. [DOI] [PubMed] [Google Scholar]
- ROSEN F., HARDING H. R., MILHOLLAND R. J., NICHOL C. A. GLUCOCORTICOIDS AND TRANSAMINASE ACTIVITY. VI. COMPARISON OF THE ADAPTIVE INCREASES OF ALANINE- AND TYROSINE-ALPHA-KETOGLUTARATE TRANSAMINASES. J Biol Chem. 1963 Nov;238:3725–3729. [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Saad A. D., Moscona A. A. Cortisol receptors and inducibility of glutamine synthetase in embryonic retina. Cell Differ. 1985 Jun;16(4):241–250. doi: 10.1016/0045-6039(85)90574-3. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Schütte J., Minna J. D., Birrer M. J. Deregulated expression of human c-jun transforms primary rat embryo cells in cooperation with an activated c-Ha-ras gene and transforms rat-1a cells as a single gene. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2257–2261. doi: 10.1073/pnas.86.7.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemshedini L., Knauthe R., Sassone-Corsi P., Pornon A., Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991 Dec;10(12):3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. Kinetics of steroid induction and deinduction of tyrosine aminotransferase synthesis in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2007–2011. doi: 10.1073/pnas.72.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Ben-Dror I., Havazelet N., Fox L. E. Molecular control of glutamine synthetase expression in the developing retina tissue. Dev Dyn. 1993 Apr;196(4):276–282. doi: 10.1002/aja.1001960410. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Cohen-Kupiec R., Degenstein L., Moscona A. A. Expression of v-src in embryonic neural retina alters cell adhesion, inhibits histogenesis, and prevents induction of glutamine synthetase. Mol Cell Biol. 1991 Oct;11(10):5275–5284. doi: 10.1128/mcb.11.10.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Moscona A. A. Accumulation of c-src mRNA is developmentally regulated in embryonic neural retina. Mol Cell Biol. 1986 Nov;6(11):4109–4111. doi: 10.1128/mcb.6.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Moscona A. A. Developmental regulation of glutamine synthetase and carbonic anhydrase II in neural retina. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Fox L. L., Degenstein L., Moscona A. A. Cell contacts are required for induction by cortisol of glutamine synthetase gene transcription in the retina. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: modulation of enzyme production by anti-inflammatory steroids, mitotic inhibitors, and cyclic nucleotides. Cell. 1976 Jun;8(2):271–281. doi: 10.1016/0092-8674(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Young A. P. A single upstream glucocorticoid response element juxtaposed to an AP1/ATF/CRE-like site renders the chicken glutamine synthetase gene hormonally inducible in transfected retina. J Biol Chem. 1991 Dec 25;266(36):24332–24338. [PubMed] [Google Scholar]
- Zhang H., Li Y. C., Young A. P. Protein kinase A activation of glucocorticoid-mediated signaling in the developing retina. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3880–3884. doi: 10.1073/pnas.90.9.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Young A. P. Exogenous, but not endogenous, glucocorticoid receptor induces glutamine synthetase gene expression in early stage embryonic retina. J Biol Chem. 1993 Feb 5;268(4):2850–2856. [PubMed] [Google Scholar]
- Zhang X. K., Dong J. M., Chiu J. F. Regulation of alpha-fetoprotein gene expression by antagonism between AP-1 and the glucocorticoid receptor at their overlapping binding site. J Biol Chem. 1991 May 5;266(13):8248–8254. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]




