Abstract
PURPOSE
The concept of mild cognitive impairment (MCI) has recently been introduced into the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as mild neurocognitive disorder, making it a formal diagnosis. We investigated the prognostic value of such a diagnosis and analyzed the determinants of the future course of MCI in the AgeCoDe study (German Study on Ageing, Cognition, and Dementia in Primary Care Patients).
METHODS
We recruited 357 patients with MCI aged 75 years or older from primary care practices and conducted follow-up with interviews for 3 years. Depending on the course of impairment over time, the patients were retrospectively split into 4 groups representing remittent, fluctuating, stable, and progressive courses of MCI. We performed ordinal logistic regression analysis and classification and regression tree (CART) analysis.
RESULTS
Overall, 41.5% of the patients had remission of symptoms with normal cognitive function 1.5 and 3 years later, 21.3% showed a fluctuating course, 14.8% had stable symptoms, and 22.4% had progression to dementia. Patients were at higher risk for advancing from one course to the next along this spectrum if they had symptoms of depression, impairment in more than 1 cognitive domain, or more severe cognitive impairment, or were older. The result on a test of the ability to learn and reproduce new material 10 minutes later was the best indicator at baseline for differentiating between remittent and progressive MCI. Symptoms of depression modified the prognosis.
CONCLUSIONS
In primary care, about one-quarter of patients with MCI have progression to dementia within the next 3 years. Assessments of memory function and depressive symptoms are helpful in predicting a progressive vs a remittent course. When transferring the concept of MCI into clinical diagnostic algorithms (eg, DSM-5), however, we should not forget that three-quarters of patients with MCI stayed cognitively stable or even improved within 3 years. They should not be alarmed unnecessarily by receiving such a diagnosis.
Keywords: mild cognitive impairment, primary care, dementia, Alzheimer disease, disease course, prognosis, practice-based research
INTRODUCTION
Mild cognitive impairment (MCI) is a common condition in the elderly with a prevalence of 16.0% in individuals without dementia1 and an incidence rate of 63.6 (per 1,000 person-years).2 It is considered to be a transitional state between normal and pathologic cognitive decline. MCI is defined by a cognitive performance below that expected for age and educational attainment, but above a pathologic level as in early dementia. Patients show essentially normal functional activities.
Winblad et al3 classified the clinical presentations of MCI into subtypes according to the impaired cognitive domains, such as memory, orientation, intellectual abilities, and higher cortical functioning. Taking into account memory deficits and the number of cognitive domains impaired, MCI subtypes have been classified as follows: amnestic single domain (impairment of memory only), amnestic multidomain (impairment of memory and 1 or more other domains), nonamnestic multidomain (no memory impairment, but impairment of more than 1 of the other domains), and nonamnestic single domain (no memory impairment, but impairment of 1 of the other domains).4,5
Until now, MCI has been a concept mainly applied in research; however, MCI has recently been added as the diagnosis of mild neurocognitive disorder to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).6 This transition from research to clinical practice aims at recognizing the substantial clinical needs of affected individuals. At the same time, it carries the risk of causing uncertainty and overtreatment of patients with MCI who may never have progression to dementia. When patients worried about their cognition consult their general practitioner, he/she will need more information on the prognostic value of such a diagnosis and on the determinants of its future course; however, research thus far has found MCI to be a condition with an unclear prognosis.7
MCI can take 4 courses, ranked by increasing severity: remittent (with recovery to normal cognitive function), fluctuating (changing between MCI and normal cognitive function), stable (impairment at each assessment that neither worsens to dementia nor improves to normal cognitive function), and progressive (development of dementia). So far, most studies have focused on the progressive course and its determinants.
In this study, AgeCoDe (German Study on Ageing, Cognition and Dementia in Primary Care Patients), we investigated determinants of the future course of MCI in primary care patients. We calculated a decision tree with factors that differentiate best between the 4 MCI courses, which will help general practitioners counsel patients about prognosis. We focused on factors that are inexpensive and, in principle, accessible in general practice.
METHODS
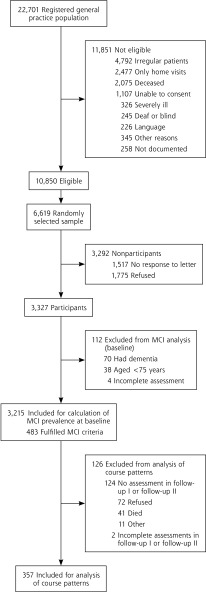
Our study methods have been described elsewhere.8 In brief, we recruited 3,327 primary care patients in 6 German cities in 2003–2004. Inclusion criteria were an age of 75 years or older, absence of dementia, and at least 1 contact with the general practitioner within the last 12 months. Exclusion criteria were consultations only by home visits, residence in a nursing home, severe illness the practitioner would deem fatal within 3 months, insufficient facility in German, deafness, blindness, inability to consent, and not being a regular patient of the practice. The study was approved by the local ethics boards of all participating centers. Written informed consent was obtained from all patients.*
Assessment Procedures
Trained interviewers (physicians or psychologists) visited the patients at their homes and carried out assessments at baseline and 18 and 36 months later. Neuropsychological assessment was based on 4 instruments for the diagnosis of dementia: (1) the Structured Interview for Diagnosis of Dementia of Alzheimer type, Multi-infarct Dementia, and Dementia of other Aetiology according to the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised (DSM-III-R), Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), and International Classification of Diseases, 10th Revision (ICD-10) (SIDAM; range = 0 to 55),9 (2) a screening test for cognitive impairment, the Mini-Mental State Examination (range = 0 to 30),10 (3) the verbal fluency subtest (1 point for each named animal within 60 seconds), word list memory subtest (range = 0 to 30), word list delayed recall subtest (range = 0 to 10), and word list recognition subtest (range = 0 to 10) of the Neuropsychological battery of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD),11 and (4) the clock-drawing test (range = 0 to 10),12 a screening test for dementia. For all above cognitive scales, a higher score indicates a better cognitive performance.
We measured depression with the short version of the Geriatric Depression Scale (range = 0 to 10; higher score indicates more severe depressive symptoms)13; activities of daily living with the SIDAM activities of daily living scale (range = 0 to 14; higher score indicates better performance)9; and instrumental activities of daily living with the scale of Lawton and Brody (range = 0 to 8; higher score indicates better performance).14 We classified education according to CASMIN criteria (Comparative Analysis of Social Mobility in Industrial Nations; categories = low, middle, high education).15 Additionally, patients were asked whether they had problems with mobility, vision, and hearing (categorized as yes or no for each). After each assessment, the interviewer rated the patient’s severity of cognitive decline on the Global Deterioration Scale (GDS) of Reisberg et al (range = 1 to 7; higher score indicates more severe impairment).16 A blood sample was taken to determine whether patients carried the apolipoprotein E ε4 allele (ApoE ε4).
Definition of Cases
We diagnosed MCI by applying consensus criteria proposed by the International Working Group on Mild Cognitive Impairment.3 These criteria have been shown to be superior to former MCI definitions for predicting dementia in general practice.17 They include (1) absence of dementia, (2) evidence of cognitive decline, from a self-rating or informant report and impairment on objective cognitive tasks and/or evidence of decline over time on objective cognitive tasks, and (3) preserved baseline activities of daily living and minimal impairment in complex instrumental functions. Dementia at baseline was excluded by an interviewer-rated GDS score of 4 or higher. Evidence of cognitive decline was defined as a SIDAM score more than 1 standard deviation below the age- and education-specific norm8 in 1 of the 4 cognitive domains it assesses (memory, orientation, intellectual abilities, and higher cortical functioning).
We categorized the patients into the 4 MCI subtypes based on their objective cognitive impairment in the cognitive domains measured by the SIDAM. Individuals with impairment in only the memory domain were classified as having amnestic single-domain MCI. Nonamnestic single-domain MCI was diagnosed only if a single domain other than memory was impaired. If at least 2 domains other than memory showed objective impairment, patients received a diagnosis of nonamnestic multidomain MCI. Amnestic multidomain MCI was diagnosed if memory and at least 1 other domain were impaired.
Dementia was considered in a patient at the first and second follow-ups based on SIDAM score, an interviewer-rated GDS of at least 4, or both. A definitive diagnosis of dementia was made in a consensus conference with the interviewer and an experienced geriatrician or geriatric psychiatrist according to the set of criteria in the DSM-IV, which are implemented as a diagnostic algorithm in the SIDAM, and taking into account all other information documented. We included all patients with an MCI diagnosis at baseline who had complete SIDAM scores at each assessment or a diagnosis of dementia at any follow-up (Figure 1).
Figure 1.
Sampling frame and sample.
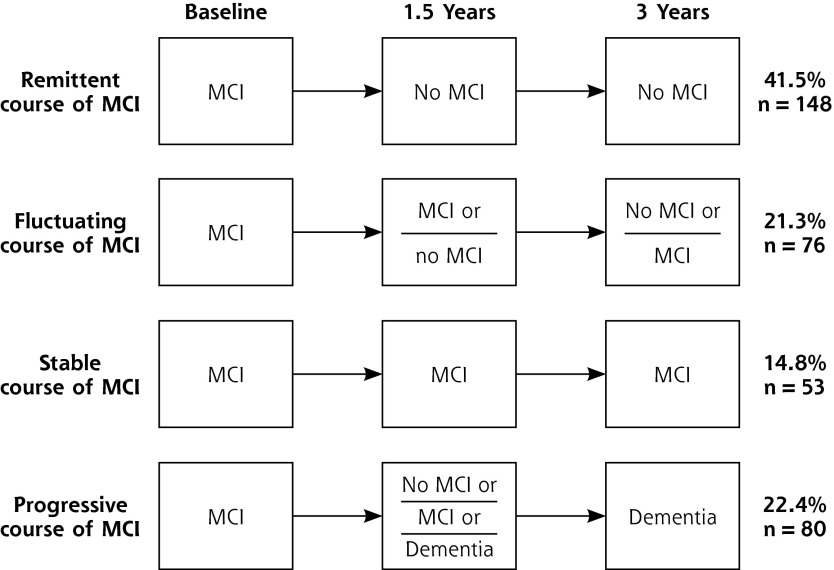
After follow-up, all patients were allocated to 1 of the 4 possible courses of MCI: remittent, fluctuating, stable, or progressive. The approach used to define these courses is shown in Figure 2.
Figure 2.
Definitions and prevalences of MCI courses.
Data Analysis
We performed statistical analyses with SPSS for Windows, versions 15 and 16 (SPSS Inc). All possible determinants of courses derived at baseline were analyzed in bivariate analyses and multivariate stepwise ordinal logistic regression analyses with the 4 possible courses of MCI as the target variable. Additionally, we performed classification and regression tree (CART) analysis to identify predictors that can discriminate between the courses of MCI. The CART method is based on recursive partitioning analysis18; the aim is to form prediction rules by constructing binary trees. Splitting rules are used as criteria to select the best split at each node; in this analysis, we used the Gini index of diversity as a measure of node Impurity as a splitting rule. A 10-fold cross-validation was used to accurately assess its goodness of fit. CART analysis has several advantages over traditional methods, including logistic regression models. It is nonparametric; no assumptions are made regarding the underlying distribution of values of the discriminator with respect to predictor variables. It can handle numerical data that are highly skewed or multimodal, including categorical predictors. CART is often able to uncover complex interactions or patterns between predictors that may be difficult or impossible to uncover using traditional multivariate techniques. CART also produces trees that are relatively simple for nonstatisticians to interpret.
RESULTS
The characteristics of the patients at baseline are given in Table 1. We excluded from analyses 126 (26%) of 483 patients at baseline because they dropped out or had insufficient data to determine the presence of MCI.
Table 1.
Patient Characteristics
| Characteristic | Included Patients | Excluded Patients | P Value |
|---|---|---|---|
| Number | 357 | 126 | |
| Age, mean (SD), y | 79.9 (3.8) | 80.4 (4.1) | .24 |
| Female sex, No. (%) | 234 (65.5) | 88 (69.8) | .38 |
| Education level (CASMIN), % | .02 | ||
| Low | 40.1 | 51.6 | |
| Medium | 44.3 | 38.9 | |
| High | 15.7 | 9.5 | |
| SIDAM score, mean (SD) | 44.8 (5.2) | 43.3 (4.9) | .006 |
| Global Deterioration Scale cognitive impairment, % | .18 | ||
| No | 24.4 | 16.7 | |
| Questionable | 29.4 | 30.2 | |
| Little | 46.2 | 53.2 | |
| Mini-Mental State Examination score, mean (SD) | 26.1 (2.2) | 25.8 (2.1) | .25 |
| ApoE ε4 carrier, % | 27.1 | 20.3 | .14 |
| MCI subtype by Winblad et al3, No. (%) | .18 | ||
| Nonamnestic single domain | 217 (60.8) | 64 (50.8) | |
| Amnestic single domain | 46 (12.9) | 19 (15.1) | |
| Nonamnestic multidomain | 38 (10.6) | 21 (16.7) | |
| Amnestic multidomain | 56 (15.7) | 22 (17.5) |
ApoE ε4 = apolipoprotein E ε4 allele; CASMIN = Comparative Analysis of Social Mobility in Industrial Nations; MCI = mild cognitive impairment; SIDAM = Structured Interview for Diagnosis of Dementia of Alzheimer type, Multi-infarct Dementia, and Dementia of other Aetiology according to the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised (DSM-III-R), Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), and International Classification of Diseases, 10th Revision (ICD-10).
The distribution of the patients according to the 4 courses of MCI during a mean ± standard deviation follow-up time of 2.94 ± 0.44 years is displayed in Figure 2.
Determinants of the Courses of MCI
In bivariate analyses, variables significantly associated with an increasingly severe course of MCI were the subtype (predominantly the multidomain amnestic subtype); more severe cognitive impairment detected by 4 CERAD subtests (verbal fluency, word list memory, word list delayed recall, and word list recognition) and by the clock drawing test; more depressive symptoms; reduced ability to walk; reduced SIDAM activities of daily living score; reduced ability to use transportation; and reduced ability to take responsibility for medication and finances (for all P <.001), as well as ApoE4 ε4 carrier status (P = .001) and reduced ability to use the telephone (P = .002). There was also a significant although slightly weaker association with older age (P = .007). Sex, education, family history of dementia, vision, and hearing ability were not significantly associated with the course of MCI.
We next considered the 4 courses of MCI as an ordinal scale of increasing severity: remittent, fluctuating, stable, and progressive. Table 2 shows the results of the multivariate stepwise ordinal logistic regression analysis with the 4 courses of MCI ranked from least severe (remittent) to the most severe (progressive) defined as outcome variable. Predictors of an increasingly severe course of MCI were worse performance in delayed recall tasks (CERAD subtest word list delayed recall) and worse ability to learn new material (CERAD subtest word list memory), more depressive symptoms, multidomain subtypes of MCI, and older age. All other determinants that were significant in the bivariate analyses lost their statistical predictive validity in the multivariate approach. The odds ratios describe patients’ likelihood of advancing to the next more severe course with every additional unit in the predictor variables. For example, for age, with each additional year, the likelihood of patients moving to the next more severe course within the next 3 years rises by 6% (odds ratio = 1.06).
Table 2.
Multivariate Odds of Advancing to Next More Severe MCI Course
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Lower score in CERAD word list delayed recalla | 1.20 | 1.047–1.372 | <.0001 |
| Depression (higher score on the Geriatric Depression Scale) | 1.24 | 1.134–1.359 | <.0001 |
| MCI subtype | .001 | ||
| Nonamnestic single domain | Ref | – | |
| Amnestic single domain | 1.42 | 0.715–2.820 | |
| Amnestic multidomain | 3.38 | 1.772–6.429 | |
| Nonamnestic multidomain | 2.30 | 1.148–4.599 | |
| Poorer test result in CERAD word list memory | 1.10 | 1.018–1.183 | .004 |
| Older age in years | 1.06 | 1.002–1.128 | .04 |
CERAD = Neuropsychological battery of the Consortium to Establish a Registry for Alzheimer’s Disease; Ref = reference group; MCI = mild cognitive impairment.
Note: MCI courses in order of increasing severity were remittent, fluctuating, stable, and progressive.
The higher the score, the better the recall function.
CART Analysis
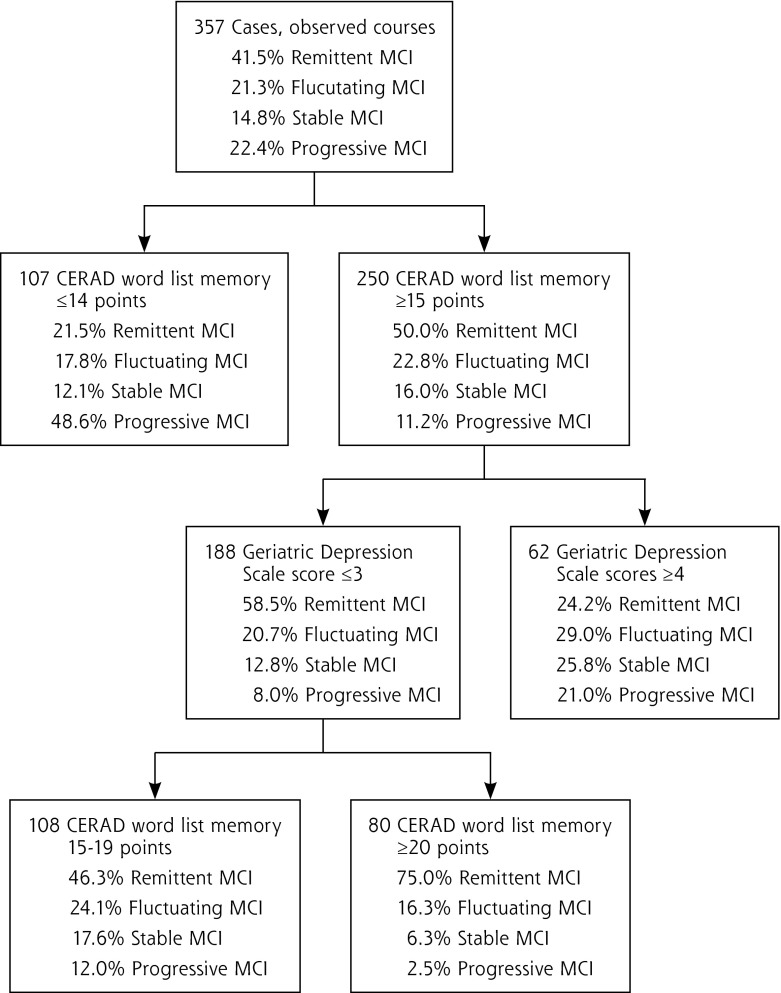
We last performed CART analysis to find out which variables best discriminate between the 4 courses of MCI. Figure 3 shows the first 3 nodes of the optimal tree found with this analysis. The first box shows the percentage of patients with each course: 41.5% had a remittent course, 21.3% a fluctuating course, 14.8% a stable course, and 22.4% a progressive course. These values represent the prognosis for each patient with MCI if no further information is available. The first node splits the sample into 2 subgroups based on the score patients achieved on the CERAD word list memory test. Among patients with a score of less than 15 on this test, the percentage with a progressive course increases from 22.4% to 48.6%. On the other hand, among patients with a score of 15 or higher, the percentage with a progressive course decreases from 22.4% to 11.2%. With further information from the Geriatric Depression Scale, we were able to identify a subgroup of patients in whom 75% have a remittent course of MCI (bottom-right box).
Figure 3.
First 3 nodes of the optimal tree found by CART analysis.
DISCUSSION
Courses of MCI
In this general practice-based study, we observed 357 patients aged 75 years or older with MCI at baseline for a mean of 3 years. Our findings illustrate that patients with MCI are a heterogeneous group with respect to disease course: 41.5% had a remittent course, 21.3% a fluctuating course, 14.8% a stable course, and 22.4% a progressive course. In comparing this study with other prospective studies of MCI, we have selected only those studies that applied the MCI criteria proposed by the International Working Group3 and were performed in primary care or population-based settings.
Progressive Course
Conversion rates from MCI to dementia have been investigated in studies with observation times of 1 to 6 years.19–23 The overall rates in those studies varied between 0.7%19 and 51.6%.23 In the majority of studies, patients with multidomain MCI were at higher risk for progression than patients with single-domain subtypes,20,24–26 but results vary regarding the exact rank order of risk associated with the subtypes.3 Furthermore, progression to dementia seems to occur primarily within the first months of observation with lower rates in later years.22,27
Our conversion rate of 22.4% in patients with a mean age of 79.9 ± 3.8 years lies between the 6.6% found in a considerably younger sample (74.6 ± 5.7 years)21 and the 30% found in an older sample (82.2 ± 5.0 years).22 This pattern underlines earlier findings of an increased risk for progression to dementia in older age.21,26,28
Remittent Course
In other studies, the rate of remittent MCI has ranged from an annual rate of 12.3% within 3.8 years to a total rate of 28.6% within 2 years.19,20,23,25,29 The highest rates of remission have been associated with nonamnestic single-domain MCI.23 The 41.5% of patients having a remittent course in our study is consistent with the data of studies having similar follow-up periods, namely, 37% within 4 years21 and an annual rate of 12.3% within 3.8 years.29
Stable Course
The stable course of MCI has been less investigated; estimated rates range from 80% in 1 year to 0% to 11% after 5 years of observation.19,20,23,25 Two studies considering subtype found conflicting results based on small numbers of patients analyzed.23,25 The rates are inconsistent, even if observation time is considered. Studies with shorter follow-up have found higher rates of stable MCI. In our study, 14.8% of patients had a stable course. Comparability with these other studies is limited, as their definitions of stable differed from ours in the number of follow-ups and observation period.
Fluctuating Course
So far, only a single other study has investigated the fluctuating course of MCI, finding that 14.1% of patients had this course within 1.5 to 6 years of follow-up, with the highest percentage among patients with nonamnestic multidomain MCI.23 In our sample, 21.3% of patients had fluctuating MCI. The lower percentage found in the aforementioned study might reflect a more progression to dementia with longer observation. Additionally, their sample included institutionalized— and hence sicker—persons. Another study did not differentiate between the stable and fluctuating course of MCI and reported that 56.5% of patients continued to meet diagnostic criteria for MCI.21 This value is higher than that for our combined group of stable and fluctuating course (36.1%), probably due to the younger age of that sample (74.6 ± 5.7 years).
Prognosis of MCI
In our study, the MCI subtypes proposed by Winblad et al3 were associated with the future course of MCI. Compared with the single-domain nonamnestic subtype, patients with multidomain amnestic MCI had a 3 times higher risk for a more severe course of MCI, followed by patients with multidomain nonamnestic MCI, whose risk was 2 times higher. Patients with the single-domain amnestic subtype did not have a significantly elevated risk. The increased risk of a more severe course among patients with the multidomain subtype supports earlier findings.20,24–26
Our CART analysis revealed that MCI subtype is not the best factor to predict the course of MCI, however. The ability to learn new material, as measured by the CERAD word list memory subtest, best differentiated between remittent and progressive MCI. This finding supports earlier findings that more severely impaired cognition predicts a progressive course28,30 and further reinforces the recommendations from the National Institute on Aging and the Alzheimer’s Association work group to use word-list learning tests for cognitive assessment.31 Further, CART analyses showed that because of strong correlations, the ability to learn new material (CERAD word list memory) is a surrogate for the performance in delayed recall tasks (CERAD word list recall).
Predictors of stable, fluctuating, or remittent courses of MCI have not been well investigated. Besides the MCI subtype, fewer chronic medical conditions and younger age predict reversion to normal cognition.32 The only factors associated with fluctuating or stable MCI so far investigated are the different MCI subtypes, as discussed above. We investigated determinants for a more severe course of MCI considering the 4 courses ranked in order of severity. We found that symptoms of depression elevate the risk of a more severe course of MCI; however, the cutoff score of 3.5 on the Geriatric Depression Scale found in the CART analysis lies within the range regarded as absence of depression33 and is clinically hardly detectable. Nonetheless, this shows that subdepressive symptoms are associated with a progressive course of MCI. These findings concur with those of some studies,21,29 whereas another study did not find this association in patients with MCI, but only in patients without MCI who had progression to dementia.34 Some investigators have suggested that depression may be an early manifestation rather than a risk factor for dementia or that depression unmasks MCI in patients with limited cognitive reserve, but is not a symptom of the neurologic condition that causes dementia.35 Age significantly influenced the course of MCI, but was not important enough to play a role in the CART analysis.
MCI in Clinical Practice
The value of diagnosing mild neurocognitive disorder, as in the DSM-5, especially in general practice, remains questionable. Patients given this diagnosis will be a heterogeneous group, and it will not have any immediate consequences in the large majority of cases. Even if there were preventive treatments, many patients would be treated unnecessarily. If the diagnosis of MCI is transferred into clinical practice within primary care, diagnostic criteria should be specified and focused on the subgroup of patients at high risk for progressive MCI. We believe that in the long term, primary care clinicians will be affected by the DSM-5 as many patients return to their general practitioner for care after a specialist diagnosis.
Strength and Limitations
This is the first study to investigate determinants of the 4 MCI courses. MCI diagnosis was based on the consensus criteria of the International Working Group,3 which have been validated within general practice.17 These criteria are very similar to the diagnostic criteria for mild neurocognitive disorder in the DSM-5.36
Limitations of our study relate to our focus on prevalent MCI. Rates for the different courses may vary depending on the time since onset. We found that 21.3% of patients switch between MCI and normal cognitive status; thus, the onset of MCI is not clearly defined. We therefore decided to investigate prevalent MCI. Because of the exclusion criteria, our study sample is representative of general practice patients who live at home and still are able to consult their practitioners in their office (a selection bias). A further limitation relates to the exclusion of some patients from analyses by attrition, mainly because of refusal and death. Compared with the included patients, the excluded patients had significantly lower SIDAM scores (43.3 ± 4.9 vs 44.8 ± 5.2) and a lower level of education. Both the selection bias and the attrition bias might have led to an overestimation of the proportion of patients with a remittent course and an underestimation of the proportion with a progressive course of MCI.
Implications
In primary care, about one-quarter of patients with MCI experience progression to dementia within the next 3 years. The performance on tasks of learning new material (CERAD subtest word list memory) and the Geriatric Depression Scale, which can detect subdepressive symptomatology, help predict the progressive versus the remittent course of MCI. Patients at high risk for progression to dementia should be monitored regularly by the general practitioner. When transferring the concept of MCI into clinical diagnostic algorithms (eg, DSM-5), however, we should not forget that three-quarters of patients with MCI stay cognitively stable or even improve within 3 years. Of those, one subgroup can be characterized well: patients with a CERAD word list memory score of at least 20 points and Geriatric Depression Scale score of 3 or less; 75% of these patients will return to normal cognitive function within the next 3 years. They should not be alarmed by receiving a clinical diagnosis of mild neurocognitive disorder.
Acknowledgments:
We thank all participating patients and their general practitioners for their good collaboration.
Footnotes
Conflicts of interest: authors report none.
The term patients is used because the participants of this study were recruited in primary care. It does not imply that the study participants were under medical treatment because of their mild cognitive impairment.
Funding support: This study and publication are part of the German Research Network on Dementia (KND) and the German Research Network on Degenerative Dementia (KNDD) and were funded by the German Federal Ministry of Education and Research (grants KND: 01GI0102, 01GI0420, 01GI0422, 01GI0423, 01GI0429, 01GI0431, 01GI0433, 01GI0434; grants KNDD: 01GI0710, 01GI0711, 01GI0712, 01GI0713, 01GI0714, 01GI0715, 01GI0716).
Previous presentations: Alzheimer’s Association 2009 International Conference on Alzheimer’s Disease (ICAD), July 11–16, 2009; Vienna, Austria. World Congress of the International Association of Gerontology and Geriatrics (IAGG), July 5–9, 2009; Paris, France. Congress of the German Association for Psychiatry and Psychotherapy (DGPPN), November 23–26. 2011; Berlin, Germany.
Members of the AgeCoDe Study Group: Wolfgang Maier and Martin Scherer (principal investigators), Hendrik van den Bussche (principal investigator 2002–2011), Heinz-Harald Abholz, Christian Brettschneider, Cadja Bachmann, Horst Bickel, Wolfgang Blank, Sandra Eifflaender-Gorfer, Marion Eisele, Annette Ernst, Angela Fuchs, Kathrin Heser, Frank Jessen, Hanna Kaduszkiewicz, Teresa Kaufeler, Mirjam Köhler, Hans-Helmut König, Alexander Koppara, Carolin Lange, Tobias Luck, Melanie Luppa, Manfred Mayer, Edelgard Mösch, Julia Olbrich, Michael Pentzek, Jana Prokein, Anna Schumacher, Steffi Riedel-Heller, Janine Stein, Susanne Steinmann, Franziska Tebarth, Michael Wagner, Klaus Weckbecker, Dagmar Weeg, Jochen Werle, Siegfried Weyerer, Birgitt Wiese, Steffen Wolfsgruber, Thomas Zimmermann.
References
- 1.Petersen RC, Roberts RO, Knopman DS, et al. ; The Mayo Clinic Study of Aging Prevalence of mild cognitive impairment is higher in men. Neurology. 2010;75(10):889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246 [DOI] [PubMed] [Google Scholar]
- 4.Petersen RCDR, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992 [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308 [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association DSM-5: The Future of Psychiatric Diagnosis. 2001. http://www.dsm5.org/Pages/Default.aspx Accessed Apr 30, 2012
- 7.Visser PJ, Brodaty H. MCI is not a clinically useful concept. Int Psychogeriatr. 2006;18(3):402–409, discussion 409–414 [PubMed] [Google Scholar]
- 8.Luck T, Riedel-Heller SG, Kaduszkiewicz H, et al. ; AgeCoDe group Mild cognitive impairment in general practice: age-specific prevalence and correlate results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe). Dement Geriatr Cogn Disord. 2007;24(4):307–316 [DOI] [PubMed] [Google Scholar]
- 9.Zaudig M, Mittelhammer J, Hiller W, et al. SIDAM—a structured interview for the diagnosis of dementia of the Alzheimer type, multi-infarct dementia and dementias of other aetiology according to ICD-10 and DSM-III-R. Psychol Med. 1991;21(1):225–236 [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198 [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165 [DOI] [PubMed] [Google Scholar]
- 12.Ihl R, Grass-Kapanke B. Test zur Früherkennung von Demenzen. Germany: Books on Demand; 2000 [Google Scholar]
- 13.Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1/2):165–173 [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186 [PubMed] [Google Scholar]
- 15.Brauns H, Steinmann S. Educational reform in France, West-Germany and the United Kingdom. ZUMA-Nachrichten. 1999;44(23):7–44 [Google Scholar]
- 16.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139 [DOI] [PubMed] [Google Scholar]
- 17.Artero S, Petersen R, Touchon J, Ritchie K. Revised criteria for mild cognitive impairment: validation within a longitudinal population study. Dement Geriatr Cogn Disord. 2006;22(5–6):465–470 [DOI] [PubMed] [Google Scholar]
- 18.Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984 [Google Scholar]
- 19.Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodaty H, Heffernan M, Kochan N, et al. Incidence, prevalence and predictors of course of mild cognitive impairment: The Sydney Memory and Ageing Study. Alzheimers Dement. 2011;7(4):S535 [Google Scholar]
- 21.Artero S, Ancelin M-L, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79(9):979–984 [DOI] [PubMed] [Google Scholar]
- 22.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. Br J Psychiatry. 2006;189(5):399–404 [DOI] [PubMed] [Google Scholar]
- 23.Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185 [DOI] [PubMed] [Google Scholar]
- 24.Palmer K, Bäckman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(7):603–611 [DOI] [PubMed] [Google Scholar]
- 25.Ritchie LJ, Tuokko H. Patterns of cognitive decline, conversion rates, and predictive validity for 3 models of MCI. Am J Alzheimers Dis Other Demen. 2010;25(7):592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baars MAE, van Boxtel MPJ, Dijkstra JB, et al. Predictive value of mild cognitive impairment for dementia. The influence of case definition and age. Dement Geriatr Cogn Disord. 2009;27(2):173–181 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell AJ, Shiri-Feshki M. Temporal trends in the long term risk of progression of mild cognitive impairment: a pooled analysis. J Neurol Neurosurg Psychiatry. 2008;79(12):1386–1391 [DOI] [PubMed] [Google Scholar]
- 28.Amieva H, Letenneur L, Dartigues J-F, et al. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement Geriatr Cogn Disord. 2004;18(1):87–93 [DOI] [PubMed] [Google Scholar]
- 29.Roberts R, Knopman D, Boeve B, et al. Outcomes of MCI: The Mayo Clinic study of aging. Alzheimers Dement. 2011;7(4):S551 [Google Scholar]
- 30.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56(1):37–42 [DOI] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olazarán J, Torrero P, Cruz I, et al. Mild cognitive impairment and dementia in primary care: the value of medical history. Fam Pract. 2011;28(4):385–392 [DOI] [PubMed] [Google Scholar]
- 33.Gauggel S, Birkner B. Validity and reliability of a German version of the Geriatric Depression Scale (GDS). [In German.] Z Klin Psychol. 1999;28(1):18–27 [Google Scholar]
- 34.Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596–1602 [DOI] [PubMed] [Google Scholar]
- 35.Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116 [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. DSM-5. 5 Washington, DC: American Psychiatric Publishing; 2013 [Google Scholar]