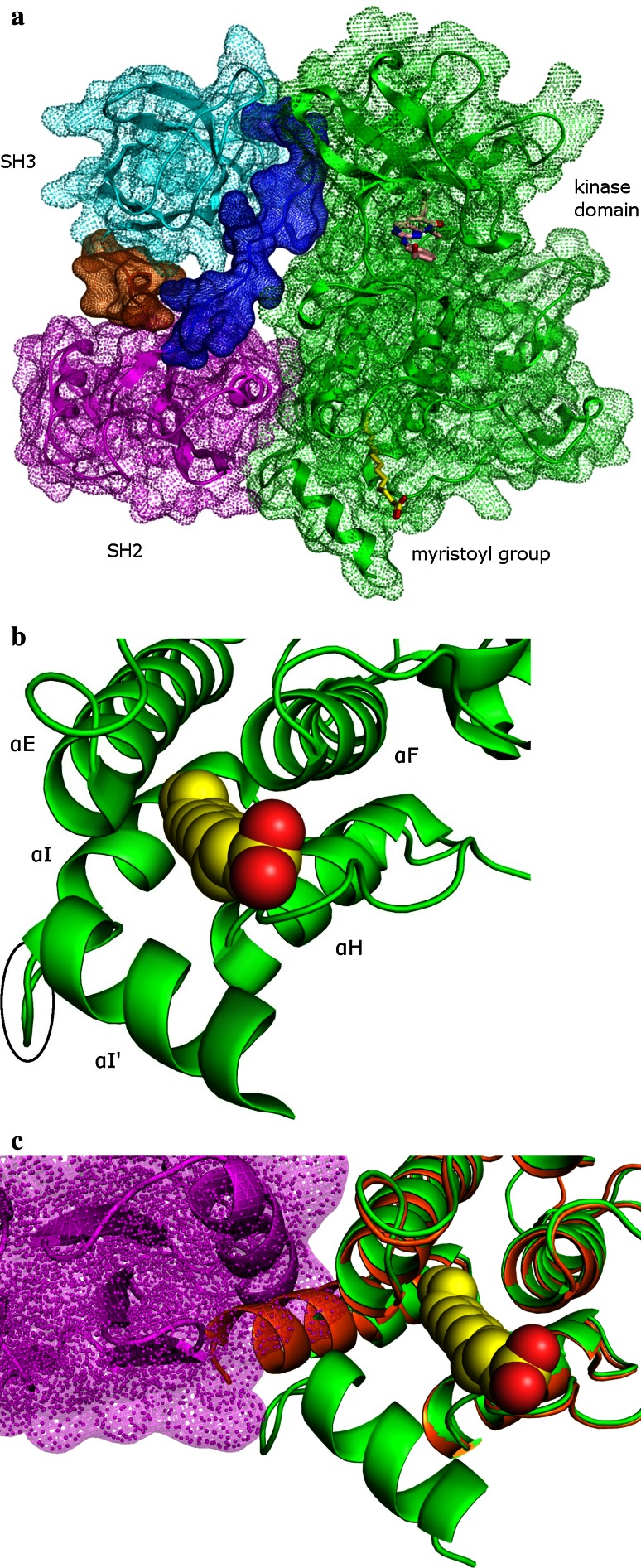
Fig. 1.
a Full view of the assembled c-Abl structure (PDB code: 1OPK). The SH3, SH2, and the kinase domains are shown in cyan, magenta, and green, respectively. The linker between the SH3 and the SH2 domains is in brown, whereas the linker between the SH2 and the kinase domains is in blue. The small molecule bound to the ATP-binding site is PD166328 [19] and is shown in pink carbon. The myristoyl group is in yellow carbon. b The myristoyl group in the c-Abl myristoyl site; the myristoyl group is shown in space-filling format and in yellow carbon. The bend between αI and αI′ (Met515–Ser519) is highlighted by a black circle. c Overlay of the assembled c-Abl structure with the myristoyl-free c-Abl kinase domain (PDB code: 1M52). The myristoyl-free c-Abl is shown in brown. The van der Waals surface of the SH2 domain is shown in magenta to illustrate the steric clash between the SH2 domain and the straight from of the C-terminal helix in the myristoyl-free c-Abl. All of the figures presented in this paper were generated using PyMol