Abstract
The choroid plexus epithelium forms the blood-cerebrospinal fluid barrier and accumulates essential minerals and heavy metals. Choroid plexus is cited as being a “sink” for heavy metals and excess minerals, serving to minimize accumulation of these potentially toxic agents in the brain. An understanding of how low doses of contaminant metals might alter transport of other solutes in the choroid plexus is limited. Using primary cultures of epithelial cells isolated from neonatal rat choroid plexus, our objective was to characterize modulation of apical uptake of the model organic cation choline elicited by low concentrations of the contaminant metal cadmium (CdCl2). At 50–1,000 nM, cadmium did not directly decrease or increase 30-min apical uptake of 10 μM [3H]choline. However, extended exposure to 250–500 nM cadmium increased [3H]choline uptake by as much as 75% without marked cytotoxicity. In addition, cadmium induced heat shock protein 70 and heme oxygenase-1 protein expression and markedly induced metallothionein gene expression. The antioxidant N-acetylcysteine attenuated stimulation of choline uptake and induction of stress proteins. Conversely, an inhibitor of glutathione synthesis l-buthionine-sulfoximine (BSO) enhanced stimulation of choline uptake and induction of stress proteins. Cadmium also activated ERK1/2 MAP kinase. The MEK1 inhibitor PD98059 diminished ERK1/2 activation and attenuated stimulation of choline uptake. Furthermore, inhibition of ERK1/2 activation abated stimulation of choline uptake in cells exposed to cadmium with BSO. These data indicate that in the choroid plexus, exposure to low concentrations of cadmium may induce oxidative stress and consequently stimulate apical choline transport through activation of ERK1/2 MAP kinase.
Keywords: choroid plexus, blood-cerebrospinal fluid barrier, cadmium, oxidative stress, choline transport
in concert, the blood-cerebrospinal fluid (CSF) barrier and blood-brain barrier compartmentalize and regulate the extracellular fluid of the central nervous system. Four choroid plexus tissues, one in each brain ventricle, collectively form the blood-CSF barrier. Each comprises a polarized epithelial monolayer with a rich supply of arterioles and capillaries. The choroid plexus epithelium forms the anatomical barrier between blood and CSF, but also secretes CSF, and selectively exchanges ions, nutrients, metabolites, xenobiotics, and growth factors into and out of the CSF via specific transport systems. Thereby, the choroid plexus regulates volume and composition of CSF, which is continuous with extracellular fluid surrounding the neurons and glia. Therefore, the choroid plexus is critical to the regulation of fluid/electrolyte balance and availability and clearance of nutrients, metabolites, or xenobiotics in the brain (1, 46).
Forming the interface of CSF and blood, the choroid plexus epithelium may respond directly to pathophysiological and physicochemical stressors originating in the central or peripheral milieus, and as the epithelium responds or adapts to stressors, solute transport may be modulated. In rats, transient forebrain ischemia markedly reduced glutamate clearance from CSF (19), and metabolic acidosis and alkalosis stimulated and inhibited, respectively, the secretory (blood-to-CSF) flux of Na (42), and hypoosmotic stress stimulated taurine efflux across the apical membrane in the isolated choroid plexus (27). Others had shown that whole body hyperthermia induced heat shock proteins Hsp70 and Hsp27 (7, 29) in the choroid plexus of the rat. We then showed in the isolated dogfish shark choroid plexus that Hsp70 induction was essential to protection of transepithelial absorption of the organic anion, 2,4-dichlorophenoxyacetic acid (2,4-D) under conditions of severe heat shock (63). However, it is possible the endogenous or xenobiotic agents transported or accumulated by choroid plexus might also elicit cellular stress and modulate transport of solute. The epithelium accumulates essential minerals, e.g., zinc and manganese, and contaminant metals, e.g., arsenite, lead, mercury, and cadmium (43, 52, 53, 59, 69, 71). As such, the choroid plexus is described as a “sink” for contaminant metals or excess essential minerals, serving to minimize accumulation of these potentially toxic agents in the brain and prevent subsequent central metal neurotoxicity (20, 69, 71). Nevertheless, a potential consequence of sequestering metals such as cadmium, which can induce cellular stress and injury, would be modulation or cessation of solute transport and, in turn, altered availability and accumulation of bioactive compounds in the brain.
Although the choroid plexus may sequester a variety of metals, few have been tested for the capacity to modulate solute transport there. We showed in the isolated dogfish shark choroid plexus that exposure to high concentrations of zinc, an essential mineral normally accumulated by the choroid plexus, decreased transepithelial absorption of 2,4-D (63). Others have elucidated modulation of solute transport by lead and manganese in great detail in cultured rat choroid plexus and in the intact plexus of rat and sheep. Lead reduces thyroid hormone thyroxine (T4) secretion into CSF by downregulating basolateral T4 uptake and transthyretin-T4 efflux into CSF (70). Downregulating low-density lipoprotein receptor via PKC-δ activation also impairs β-amyloid protein (Aβ) clearance from CSF, disrupting intracellular Aβ metabolism (5, 22). Excess manganese can increase iron levels in CSF by upregulating transferrin receptor-mediated basolateral uptake of iron and apical efflux of iron into CSF via ferroportin (64). However, investigation of modulation of solute transport in the choroid plexus by exposure to other metals has been limited.
In the present study, we used primary cultures of neonatal rat choroid plexus epithelial cells to elucidate how the contaminant metal cadmium might modulate transport of choline, an endogenous amine and model substrate for the organic cation transport system at the apical membrane of the epithelium (61, 62). Despite its toxicity, cadmium is used to manufacture common items, such as paint, polyvinyl chloride, and rechargeable batteries (2). Animal- and plant-based foods, such as oysters, clams, leafy greens, and sunflower seeds, contain measurable amounts of cadmium. Nonetheless, cigarette smoking is a prominent route of cadmium exposure, and blood cadmium concentrations in smokers may be twice those in nonsmokers (48). Not readily cleared from the body, cadmium has a biological half-life of up to 30 years (2). Acute and chronic exposure studies in rodents and rabbits has established that the choroid plexus also accumulates cadmium (53, 59, 69). In mice, 22-wk exposure to cadmium in drinking water at 10 and 100 ppm elicited dose-dependent changes indicative of cellular injury in choroid plexus epithelia, such as shortening and loss of microvilli and intracellular vacuole formation (59). Still, characterization of the effects of cadmium, particularly at low doses, on solute transport in this barrier epithelium has been limited.
We had previously established that epithelial cells isolated from neonatal rat choroid plexus that were seeded on impermeable supports proliferate and differentiate, forming confluent epithelial monolayers. These monolayers maintain morphological, biochemical, and functional polarity similar to that of the intact choroid plexus (61). In choroid plexus cells plated on impermeable supports, the ventricular (apical) membrane of the epithelium is exposed. We demonstrated that cellular uptake of choline at the apical membrane was mediated by an electrogenic facilitated diffusion, energetically but indirectly coupled to Na+ transport (62); molecular studies by Sweet et al. (51) suggested the organic cation transporter OCT2 (SLC22A2) may mediate apical choline uptake in the choroid plexus. Using primary cultures of neonatal rat choroid plexus epithelial cells, our present objective was to characterize modulation of apical choline uptake elicited by exposure to nonlethal low concentrations of cadmium, e.g., 500 nM, sufficient to induce cellular stress. On the basis of early reports of cellular injury in intact choroid plexus of cadmium-treated mice, we anticipated cadmium treatment would impair transport function. Cultured choroid plexus epithelia exposed to submicromolar cadmium mounted a cellular stress response characterized by upregulation of Hsp70, heme oxygenase-1, and metallothionein-1. However, apical choline uptake was not impaired, but instead, markedly stimulated. The results herein indicated stress modulation of apical choline uptake was consequential to induction of oxidative stress and mediated through activation of ERK1/2 MAP kinase.
MATERIALS AND METHODS
Animals and tissue harvest.
Choroid plexus tissues were harvested from 2–3-day-old Sprague-Dawley rats. Neonatal rats were obtained from timed-pregnant dams that arrived at the university vivarium at gestational day 16; dams had free access to food and water. With approval from the University Committee on Animal Resources at the University of Rochester (protocol no. 2000–126) and the Institutional Animal Care and Use Committee at Texas A&M University (protocol no. 2011-128), choroid plexus tissues were harvested from the brains of neonatal rats using ethanol-sterilized instruments. For an individual culture preparation, lateral and fourth choroid plexus tissues from 36–50 individual animals were pooled and held in chilled collection medium.
Solutions and chemicals.
Tissue collection medium consisted of DMEM/F12 (Sigma, St. Louis, MO) and penicillin (100 U/ml; Calbiochem-EMD Millipore, Billerica, MA). Dissociation buffer contained (in mM) 137 NaCl, 2.7 KCl, 0.7 Na2HPO4, 5.6 glucose, and 10 HEPES (pH 7.4) and 5 U/ml protease (Sigma) and 1,500 kU/ml DNase I (Calbiochem-EMD Millipore). Cells were preplated in penicillin-supplement DMEM/F12 with 10% Nu-Serum IV (BD Biosciences, San Jose, CA). Initial plating medium was minimum essential medium with d-valine substituted for l-valine (U.S. Biological, Swampscott, MA) with 10% Nu-Serum IV and 1.5 μM triiodo-l-thyronine, 50 ng/ml epidermal growth factor, 100 ng/ml prostaglandin E1, and 10 μM forskolin; all growth supplements were of tissue culture grade and purchased from Sigma. Maintenance medium consisted of DMEM/F12 with 5% Nu-Serum IV and the growth supplements listed above at the same concentrations. Plating and maintenance media did not contain antibiotics or fungicides. Transport of radiolabeled choline was assayed in artificial cerebrospinal fluid (aCSF) containing (in mM) 137.4 sodium, 3 potassium, 1.4 calcium, 0.8 magnesium, 0.7 phosphate, 125.4 chloride, 2 urea, 18 bicarbonate and 10 Tris-HEPES (pH 7.4) with 12 mM glucose, 10 μM unlabeled choline chloride (Tokyo Chemical Industry, Tokyo, Japan), and trace [3H]choline chloride (0.075 μCi, ∼80 Ci/mmol; Perkin-Elmer, Waltham, MA). For analysis of elemental cadmium accumulation, treated cells were rinsed with PBS (in mM: 137 NaCl, 2.7 KCl, 10 Na2HPO4, and 1.8 KH2PO4, at pH 7.4). For immunoblot analysis of protein, treated cells were rinsed with PBS, and TBS (in mM: 150 NaCl and 50 Tris·HCl, at pH 7.5) was used to wash and probe membranes with primary and secondary antibodies.
All chemicals were of analytical grade and were purchased from commercial vendors. The following specific reagents were purchased from the respective vendors: cadmium chloride (Sigma), N-acetylcysteine (Calbiochem-EMD Millipore), buthionine sulfoximine (Acros Organics, Morris Plains, NJ), and PD98059 (Calbiochem-EMD Millipore).
Choroid plexus epithelial cell isolation and primary culture.
Epithelial cells were dispersed from neonatal rat choroid plexus tissues following the protocol described previously (61) with minor modifications. Briefly, tissues were suspended in dissociation buffer, and the tissue-enzyme suspension was shaken at 37°C and triturated intermittently over a 20-min period. Aliquots of released cells were filtered through a sterile nylon cell strainer (100 μm; BD Biosciences, San Jose, CA) intermittently rinsed with penicillin-supplemented DMEM/F12 with 10% Nu-Serum IV; this also diluted the filtrate enzyme concentration. The cell suspension was centrifuged and washed once with a generous volume of penicillin-supplemented DMEM/F12. Cells then were suspended in penicillin-supplemented DMEM/F12 with 10% Nu-Serum IV and preplated in a single 35-mm Petri dish for 3.5 h at 37°C (humidified 95% air-5% CO2). Unattached cells then were collected, centrifuged, and suspended in plating medium and plated at a density of 3 × 105 cells/cm2 on impermeable supports, e.g., polystyrene tissue culture plates, and maintained at 37°C (humidified 95% air-5% CO2). Seventy-two hours after plating, unattached cells were removed, and the initial plating medium was replaced with maintenance medium; thereafter, medium was changed every 2–3 days. Cells grew to confluence as a differentiated monolayer within 6 days, and experiments were conducted 6–9 days postplating.
In vitro cadmium exposure.
Stock solutions of ∼5 mM CdCl2 in sterile ultra pure water were prepared biweekly. For cadmium exposure only during the assay of choline transport, a working stock solution of 500 μM CdCl2 in aCSF containing 10 μM choline chloride and trace [3H]choline chloride was prepared; that working stock solution was diluted serially with cadmium-free aCSF containing 10 μM choline chloride and trace [3H]choline chloride to generate transport assay buffers containing 50–1,000 nM CdCl2. For extended cadmium exposure, a working stock solution of 500 μM CdCl2 in sterile DMEM/F12 was prepared and then diluted serially with serum-free DMEM/F12 to generate treatment media containing 50–1,000 nM CdCl2. Additional test agents were added to treatment media as needed. Before initiation of experimental and control treatments with cadmium and other agents to manipulate cellular stress or signaling, all cells were incubated for 12–16 h in serum-free DMEM/F12 without growth supplements. Cells were incubated in serum-free DMEM/F12 containing 50–1,000 nM CdCl2 for up to 24 h at 37°C (humidified 95% air-5% CO2); in parallel, control cells were incubated in serum-free DMEM/F12 without cadmium or other test agents.
Radiotracer assay of apical uptake of choline.
Thirty-minute apical uptake of [3H]choline was assayed in cells plated in 96-well tissue plates, as described previously (62). To assess whether cadmium directly inhibited or stimulated choline uptake, cells were triple-rinsed in aCSF and then incubated (30 min, 37°C, 95% air-5% CO2) with 100 μl aCSF containing 10 μM unlabeled choline chloride plus trace [3H]choline chloride and 0 or 50–1,000 nM CdCl2. To assay choline uptake after extended cadmium exposure, cells were tripled-rinsed with aCSF and then incubated (30 min, 37°C, 95% air-5% CO2) with 100 μl aCSF containing 10 μM unlabeled choline chloride and trace [3H]choline chloride ± 750 μM hemicholinium-3 (HC-3); however, uptake buffer did not contain cadmium or other agents used to manipulate cellular stress or signal transduction. In all cases, uptake was terminated by removal of transport buffer and a triple rinse with ice-cold isotope-free aCSF containing 5 mM choline chloride (this also removed residual radiolabeled choline from the culture well). Cells were solubilized in 100 μl 1 M NaOH and neutralized with 100 μl 1 N HCl. Two 50-μl aliquots of the solubilized cell suspension were collected for determination of radioactivity by liquid scintillation. A third 50-μl aliquot of the cell suspension was collected to determine protein content by a Bradford assay (Bio-Rad, Hercules, CA) using BSA as a standard. Radiolabeled [3H]choline uptake was calculated as picomoles [3H]choline per milligram protein. Unless stated otherwise, apical choline uptake was measured in triplicate (triplicate measures) in at least three separate culture preparations (n = 3). Data are reported as a percentage of mediated choline uptake by control cells; means ± SE.
Analysis of cellular accumulation of cadmium.
Total cellular accumulation of elemental cadmium was determined by atomic absorption spectrometry. Cells grown in 12-well plates were incubated in 1 ml serum-free DMEM/F12 with 0 or 500 nM CdCl2 for 12 h. After treatment, medium was removed, and cells were rinsed twice with 1 ml of chilled PBS (calcium-free and magnesium-free) with 5 mM EDTA. Cells then were solubilized in 2% HNO3 (Ultrex grade) in double-distilled deionized water. From each control and experimental sample, three 12-μl aliquots of the cell suspension were collected for analysis of elemental cadmium using a Perkin-Elmer A Analyst 600 atomic absorption spectrophotometer equipped with longitudinal Zeeman background correction and a transverse heated graphite furnace (Perkin-Elmer Life and Analytical Sciences, Boston, MA). Reference solutions of cadmium containing 0, 2, 5, 10, and 20 ng/ml 2% HNO3 were analyzed to calibrate the instrument. The LOD for cadmium was 0.053 ng/ml, and the LOQ was 0.177 ng/ml. In parallel, representative cells were subject to control and cadmium-exposed conditions; these cells were then processed for determination of total cellular protein by a Bradford assay (Bio-Rad) using BSA as a standard. Total cellular accumulation of cadmium was expressed as nanograms per milligram protein. Elemental cadmium accumulation in control and cadmium-exposed cells was analyzed in three separate culture preparations (n = 3).
Lactate dehydrogenase release.
Extracellular lactate dehydrogenase (LDH) released from nontreated control cells and cadmium-exposed cells was assayed using a commercial kit (CytoTox 96 Nonradioactive Cytotoxicity Assay; Promega, Madison, WI). Cells grown in 48-well plates were incubated with 400-μl treatment medium. Maximum LDH release was determined in nontreated control cells lysed with 0.9% vol/vol Triton X-100. After treatment, a 50-μl sample from each control and test well was transferred to a well of a cell-free 96-well plate and mixed with 50-μl substrate mix. After 10-min incubation (24°C), a 50-μl stop solution was added to each well, and absorbance was recorded at 490 nm (Tecan-Infinite M200 plate reader; Morrisville, NC). Values were corrected for background absorbance, i.e., cell-free DMEM/F12. LDH release was expressed as a percentage of maximal LDH release; LDH release was measured in triplicate in at least three separate culture preparations (triplicate measures; n = 3).
Immunoblot analysis.
Cells were plated in 96-well or 48-well plates and incubated with 200 or 400 μl experimental medium. After treatment, cells were rinsed with PBS/0.5% Triton X-100 with a cocktail of phosphatase inhibitors and protease inhibitors and lysed with sample buffer (50 mM Tris·HCl at pH 6.8, 100 mM DTT, 30% vol/vol glycerol, 2% wt/vol SDS, 0.05% vol/vol Triton X-100, 0.5% wt/vol bromophenol blue) containing phosphatase/protease inhibitor cocktail. Cell lysates were heat-denatured, sonicated, and centrifuged before cellular proteins were separated by electrophoresis (10% SDS-polyacrylamide gel) and electroblotted onto polyvinylidene difluoride membrane. For analysis of hemeoxygenase-1 (HO-1), heat shock protein-70 (Hsp70), and β-actin, membranes were blocked (2 h, 24°C) with 10% nonfat dry milk (NFDM)/TBS/0.1% Tween-20 (TBS-T) and then incubated at 4°C overnight or at 24°C for 2 h in 10% NFDM/TBS-T with primary antibodies against HO-1 (rabbit polyclonal, 1:2,000; Enzo, Farmingdale, NY), Hsp70 (rabbit polyclonal, 1:1,000; Enzo), or β-actin (mouse monoclonal, 1:1,000; Sigma). Subsequently, membranes were incubated (24°C, 1.5 h) with alkaline phosphatase-conjugated secondary antibody against rabbit or mouse IgG (3:10,000; Enzo). Immunoreactivity was detected with chromogenic substrates, 5-bromo-4-chloro-3-indolyl-phosphate (BCIP), and nitro blue tetrazolium (Promega) and digitally analyzed (Alpha Innotech FluoroChem gel documentation system). For analysis of phosphorylated ERK1/2 and total ERK1/2, membranes were blocked with 5% BSA/0.1% Tween-20/TBS (2 h, 24°C) then incubated (18 h, 4°C) in buffer of similar composition with primary rabbit polyclonal antibody against phosphorylated ERK1/2 (1:1,000; Cell Signaling Technology, Danvers, MA) or total ERK1/2 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes then were incubated (1.5 h, 24°C) with horseradish peroxidase-labeled secondary antibody against rabbit IgG (1:1,000; Enzo) then treated (5 min, 24°C) with luminol-based chemiluminescent substrate (Immobilon Western, Millipore); immunoreactivity was visualized on X-ray film. For each sample, relative area and intensity of individual HO-1, Hsp70, phosphorylated and total ERK1/2, and β-actin bands were quantitated by densitometry (Alpha Innotech FluoroChem analysis software). Accumulation of HO-1 and Hsp70 was normalized to that of β-actin; accumulation of phosphorylated-ERK1/2 was normalized to that of total ERK1/2.
Quantitative real-time RT-PCR (qRT-PCR) analysis of gene expression.
Cells were plated in 12-well culture plates and incubated with 1 ml experimental medium. After treatment, total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA). Per manufacturer's instructions, cells were disrupted in 500 μl lysis buffer, and the suspension was homogenized by centrifugation through a QIAshredder. Lysates were cleared with EtOH, applied to the RNeasy Spin Column, and treated with DNase (Qiagen) before final elution in RNase-free water. RNA was evaluated for quality and contamination based on Abs260 nm:Abs280 nm and Abs260 nm:Abs230 nm ratios determined by nanospectrophotometry and for integrity using an Agilent 2100 Bioanalyzer (Santa Clara, CA) and an Agilent RNA 6000 Nano Kit (Santa Clara, CA). First-strand cDNA was synthesized (Superscript III First-Strand Synthesis System SuperMix; Invitrogen, Carlsbad, CA) from samples with RNA integrity numbers >8. Gene expression in each cDNA sample was analyzed in triplicate by qRT-PCR with SYBR Green detection (iQ SYBR Green Supermix; Bio-Rad) in a single-color real-time detection system (Bio-Rad MyQ). Copy numbers for metallothionein (Mt-1), β-actin, and GAPDH mRNA were determined; copy number of Mt-1 mRNA was normalized to that of β-actin and GAPDH mRNA expression. Initial denaturation: 10 min, 95°C; amplification/quantification (45 cycles): 15 s, 95°C; 30 s, 60°C; melt curve: 55°C–95°C. Primers were designed (Primer Express software, PE Applied Biosystems, Foster City, CA) on the basis of GenBank sequences for rat β-actin (NM_031144), GAPDH (NM_017008), and Mt-1 (NM-138826) and synthesized by DNA Technologies (Coralville, IA). Primer sequences were as follows: β-actin, forward: 5′-ATGGTGGGTATGGGTCAG-3′, reverse: 5′-TACTTCAGGGTCAGGATGC-3′; GAPDH: forward: 5′-ATGACTCTACCCACGGC-3′, reverse: 5′-ACTCAGCACCAGCATCA-3′; and Mt-1: forward: 5′-CACCGTTGCTCCAGATTCA-3′; reverse: 5′-CAGCAGCACTGTTCGTCA-3′. Mt-1 mRNA was analyzed in control and cadmium-exposed cells in three separate primary culture preparations (n = 3).
Statistics.
Results shown are expressed as means ± SE. Control and experimental means were compared by two-tailed Student's paired t-test or ANOVA with the appropriate post hoc test. Differences were deemed significant at P ≤ 0.05.
RESULTS
Time- and concentration-dependent effects of cadmium on apical choline uptake in primary cultures of neonatal rat choroid plexus epithelial cells.
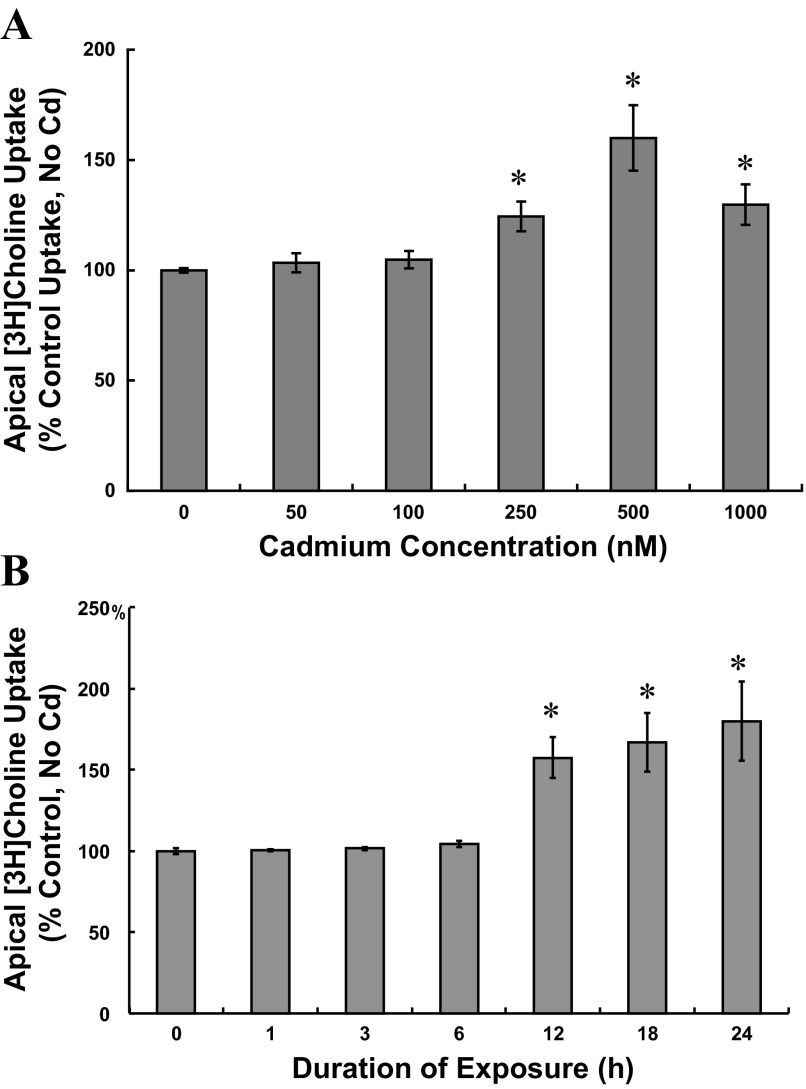
To assess the direct effects of cadmium (CdCl2) on apical choline uptake in choroid plexus epithelial (CPE) cells, we exposed cells to cadmium only during the transport assay. Total apical uptake of 10 μM [3H]choline was assayed (30 min) in aCSF containing 0–1,000 nM CdCl2 (Table 1). In the absence of cadmium, total choline uptake was 2,928.8 ± 264.1 pmol choline/mg protein (n = 3), and at concentrations up to 1,000 nM, cadmium did not alter uptake (P > 0.78). These data indicated cadmium did not directly alter apical choline uptake. To evaluate the effects of extended cadmium exposure on apical choline uptake, we incubated cells for up to 24 h in serum-free medium without cadmium (control) or with 50–1,000 nM CdCl2 (Fig. 1) and then assayed 30-min mediated apical uptake of choline (10 μM [3H]choline ± 750 μM hemicholinium-3, HC-3) in cadmium-free buffer. Compared with control conditions (No serum/No Cd), 24-h exposure to 50 or 100 nM cadmium did not alter apical choline uptake, whereas 24-h exposure to 250, 500, and 1,000 nM cadmium increased uptake by 25%, 60%, and 30% (Fig. 1A; P < 0.002 vs. No serum/No Cd). To assess time-dependent effects of cadmium on choline uptake, we exposed cells to 500 nM CdCl2 for 1–24 h (Fig. 1B). Six-hour exposure to 500 nM cadmium was without effect (P > 0.30 vs. No serum/No Cd); however, choline uptake increased by 60% after 12-h exposure and by 65% and 80% after 18-h and 24-h exposure to cadmium. In absence of cadmium, serum deprivation alone could have induced stress and, consequently, stimulated choline uptake. We compared 30-min apical choline uptake (10 μM [3H]choline ± 750 μM HC-3) in cells incubated for 12 h in DMEM/F12 with 5% NuSerum and then for a subsequent 12-h period with fresh medium with 5% NuSerum to choline uptake in cells incubated in a similar manner for two consecutive 12-h periods in DMEM/F12 without serum. Choline uptake in serum-deprived cells (No CdCl2) was comparable to that in cells incubated for the same duration with 5% NuSerum (No serum/No Cd: 3,876 ± SE 113 vs. 5% NuSerum/No Cd: 3,858 ± SE 197 pmol choline·mg protein−1·30 min−1; n = 3; P > 0.87). By atomic absorption spectrophotometry, we analyzed total cadmium accumulation in CPE cells incubated for 12 h in serum-free medium with 500 nM CdCl2 vs. No CdCl2, and normalized cadmium content to mg total cell protein (n = 3). Total cadmium accumulation in CPE cells incubated with the metal was 266.47 ± SE 8.88 ng/mg protein; background levels of cadmium in nontreated controls was 2.17 ± SE 0.37 ng/mg protein (n = 3; P < 0.0004). Thus, under these experimental conditions, CPE cells accumulated the heavy metal. As a general index of cytotoxicity, by colorimetric assay, we measured LDH release from cells after 12-h exposure to 500 nM CdCl2 in serum-free medium. LDH release was expressed as a percentage of maximal LDH release from control cells (No serum/No Cd; n = 5). LDH release from control cells was 8.46% ± SE 0.77% of maximal LDH release, and LDH from cadmium-exposed cells was 12.04 ± 2.95 of maximal LDH release (P > 0.09 vs. control). On the basis of this modest increase in LDH release induced by 500 nM cadmium at 12 h, we proceeded to elucidate modulation of apical choline uptake in CPE cells exposed to not more than 500 nM CdCl2 for durations not exceeding 12 h.
Table 1.
Acute effects of cadmium on 30-min apical uptake of 10 μM [3H]choline in cultured choroid plexus epithelial cells
| Cadmium Concentration, nM | [3H]Choline Uptake, pmol·mg protein−1·30 min−1 |
|---|---|
| 0 (Control) | 2928.8 ± 264.1 |
| 50 | 3121.7 ± 174.0 |
| 100 | 3008.9 ± 280.5 |
| 250 | 3030.8 ± 232.8 |
| 500 | 2947.2 ± 211.0 |
| 1,000 | 2923.9 ± 200.1 |
Values are expressed as means ± SE, using triplicate measures; n = 3 culture preparations. Choroid plexus cells were plated on impermeable supports and exposed to cadmium only during the transport assay. Cells were incubated at 37°C for 30 min in artificial cerebrospinal fluid (10 mM Tris-HEPES, pH 7.4) with 10 μM [3H]choline chloride and 0–1000 nM cadmium chloride. At the tested concentrations, cadmium did not alter choline uptake; P > 0.62 vs. control uptake in the absence of cadmium.
Fig. 1.
Effects of cadmium exposure on apical uptake of 10 μM [3H]choline in cultured choroid plexus epithelial cells plated on impermeable supports. A: concentration-dependent effects of cadmium on apical choline uptake were examined in cells incubated for 24 h in serum-free medium without cadmium (control) or with 50–1,000 nM CdCl2. B: time-dependent effects of cadmium on apical choline uptake were examined in cells incubated for 0–24 h in serum-free medium without cadmium (control) or with 500 nM CdCl2. After treatment, cells were rinsed and incubated (30 min, 37°C) in cadmium-free artificial cerebrospinal fluid (CSF; 10 mM Tris-HEPES, pH 7.4) with 10 μM [3H]choline chloride ± 750 μM hemicholinium-3. [3H]choline uptake was expressed as a percentage of uptake in control cells incubated for 24 h in serum-free medium without cadmium. For A and B, values are expressed as means ± SE; n = 3. *Significantly different than nontreated 24-h controls (P < 0.05).
Induction of the cellular stress response in cadmium-exposed CPE cells.
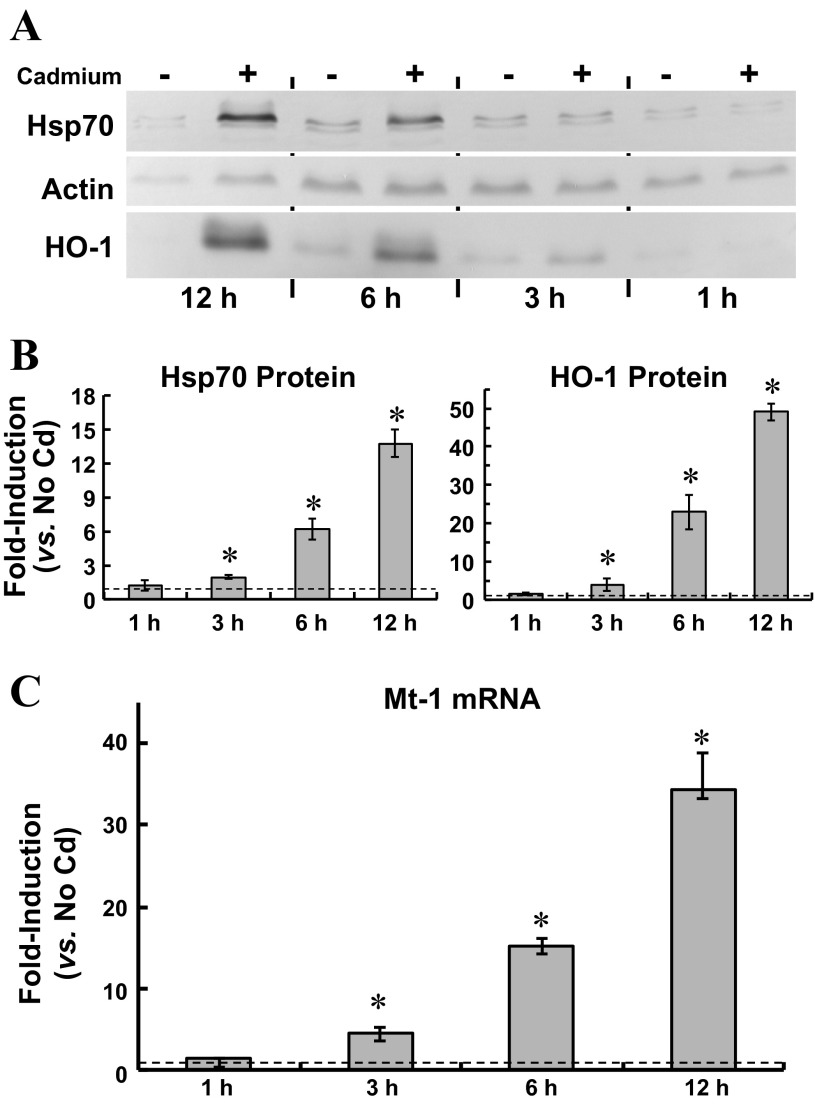
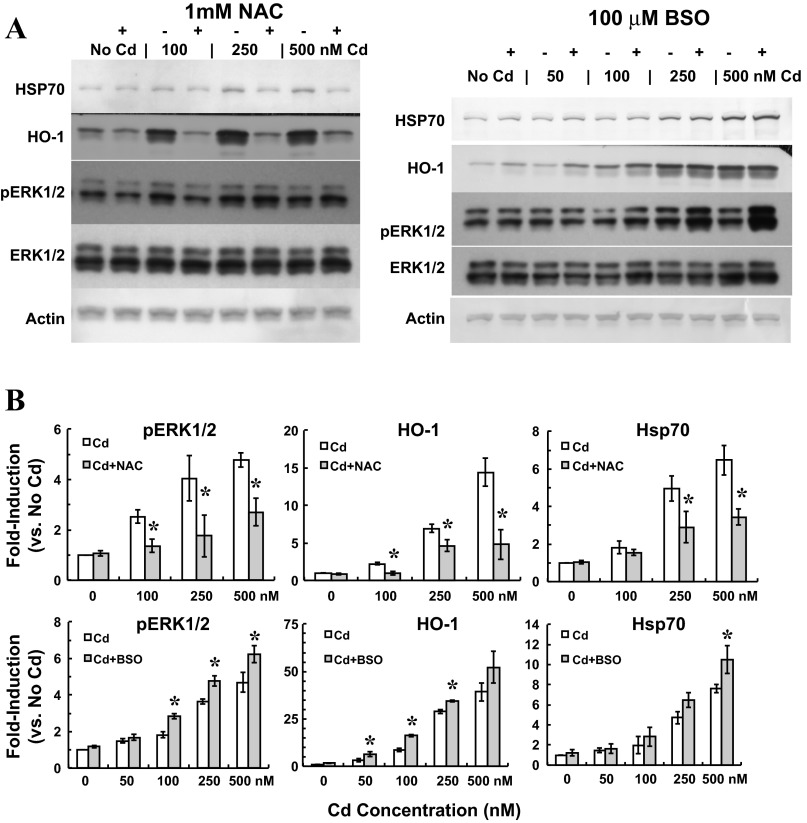
In choroid plexus, solute transport can be modulated as the epithelium mounts a cellular stress response (63). Cadmium is among the abiotic stressors that elicit the cellular stress response in vivo and in vitro (30), and in cadmium-exposed CPE cells, modulation of apical choline uptake might have been consequential to a cellular stress response. Thus, we characterized cellular stress in cadmium-exposed cells. Induction of heat shock protein-70 is a hallmark of the cellular stress response (30). Furthermore, the cellular stress response elicited by cadmium and other non-Fenton metals is characterized by induction of hemeoxygenase-1 (HO-1) and Mt-1 (18, 58). Thus, we examined induction of Hsp70, HO-1, and MT-1 in CPE cells exposed to 500 nM CdCl2 for 0–12 h (Fig. 2). Time-dependent accumulation of Hsp70 and HO-1 was assayed by immunoblot analysis (Fig. 2A, representative blot; n = 3). Compared with that in control cells (No Cd, 12 h), total Hsp70 in cadmium-exposed cells increased by twofold at 3 h (1.9 ± 0.2) and by 13-fold (13.7 ± 1.9) at 12 h. Similarly, in cadmium-exposed cells total HO-1 doubled (2.8 ± 0.6) at 3 h and progressively increased by nearly 50-fold (49.1 ± 2.3) at 12 h. Induction of metallothionein was evaluated by quantitative real-time RT-PCR (qRT-PCR) analysis of Mt-1 mRNA (Fig. 2B); Mt-1 mRNA increased by 5-fold at 3 h and 35-fold at 12 h. Collectively, these data indicated cadmium elicited a cellular stress response.
Fig. 2.
Induction of heat shock protein 70 (Hsp70) and heme oxygenase-1 (HO-1) protein expression and metallothionein (Mt-1) gene expression in cultured choroid plexus epithelial cells exposed for 12 h to 500 nM CdCl2 in serum-free medium. A and B: at 1, 3, 6, and 12 h, representative cells were lysed for determination of actin, Hsp70, and HO-1 proteins by immunoblot analysis. A: representative immunoblot of 12-h accumulation of Hsp70, HO-1, and β-actin in cadmium-exposed cells; immunoreactivity of each protein with the respective primary antibody was visualized by colorimetric detection using alkaline phosphatase-conjugated secondary antibody and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitro blue tetrazolium (NBT) reagent. B: summarized data for time-dependent induction of Hsp70 and HO-1 proteins in three separate culture preparations exposed to cadmium. For each control and experimental sample, pixel densities of Hsp70 and HO-1 bands were normalized to that of β-actin; fold-induction was calculated as a ratio of normalized Hsp70 or HO-1 expression to normalized Hsp70 or HO-1 expression in nontreated controls, i.e., no cadmium (n = 3; means ± SE). *Significantly different than nontreated 12-h controls (P < 0.05). C: induction of Mt-1 mRNA in cells exposed to 500 nM CdCl2 for 12 h was analyzed by quantitative real-time RT-PCR with SYBR Green detection and normalized to actin and GAPDH mRNA expression. Fold induction was calculated as the ratio of normalized Mt-1 mRNA expression in cadmium-exposed cells to that in cells incubated for 12 h in cadmium-free medium (n = 3; means ± SE). *Significantly different than nontreated 12-h controls (P < 0.05).
Manipulation of oxidative stress and modulation of apical choline uptake in cadmium-exposed CPE cells.
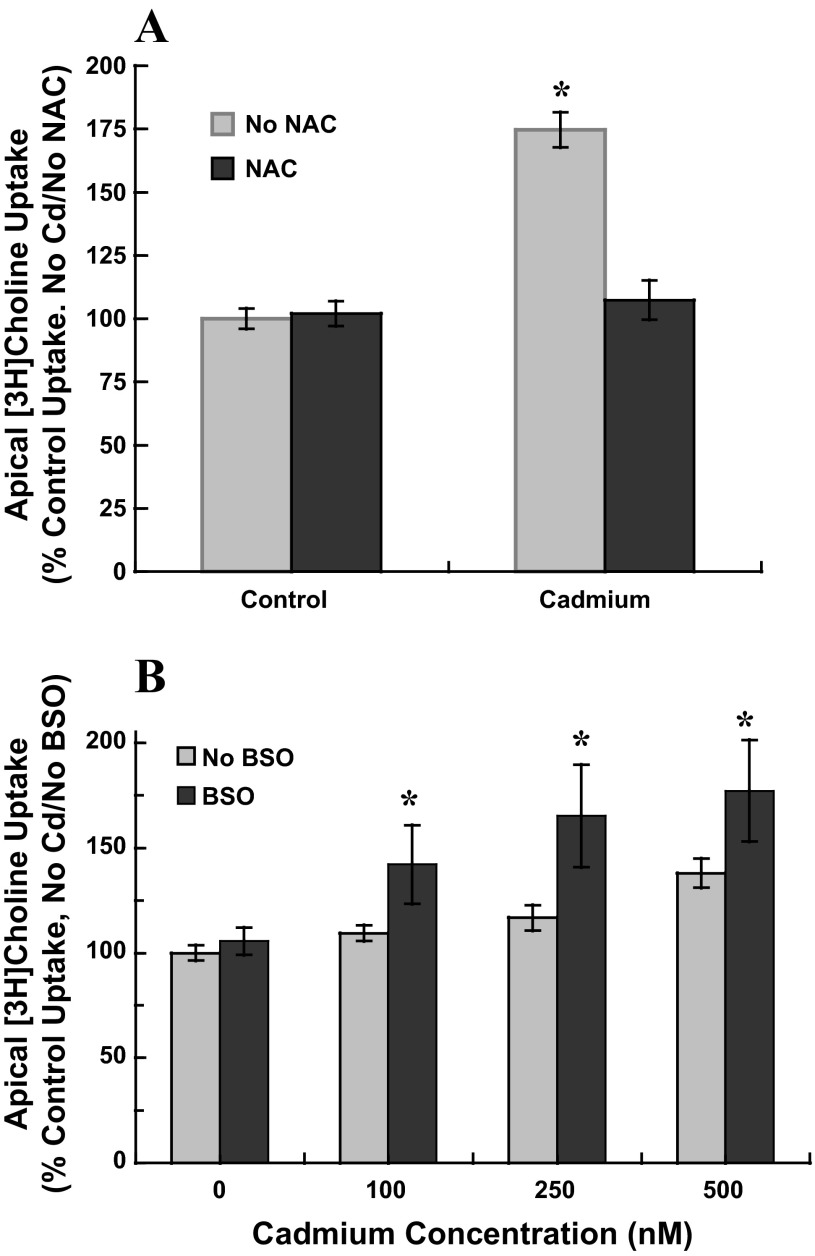
Cadmium does not directly generate free radicals but can indirectly increase intracellular accumulation of free radicals and induce oxidative stress. Cadmium can displace the Fenton metals iron and copper that may then generate free radicals by the Fenton reaction (10). Total reactive oxygen species (ROS) also may increase subsequent to cadmium inhibition of complex III of the electron transport chain (65). Glutathione (GSH) and other endogenous scavengers can be depleted as they bind cadmium and are transported out of the cell (31); subsequently, additional ROS may accumulate in the cell. If oxidative stress were a mediating factor in stimulation of apical choline uptake in cadmium-exposed cells, attenuation of oxidative stress would suppress stimulation of uptake and exacerbation of oxidative stress would augment stimulation. To attenuate putative induction of oxidative stress by cadmium, we used N-acetylcysteine (NAC), a thiol antioxidant and precursor to GSH. We used l-buthionine-sulfoximine (BSO), which decreases intracellular GSH by inhibiting glutamate cysteine ligase the rate-limiting enzyme in GSH synthesis and is known to exacerbate cadmium-induced oxidative stress (26). We exposed CPE cells for 12 h to submicromolar CdCl2 without or with NAC or BSO and then assayed 30-min apical choline uptake (10 μM [3H]choline ± 750 μM HC-3) in buffer free of cadmium, NAC, and BSO (Fig. 3). NAC and BSO were tested in separate culture preparations. We exposed cells for 12 h to 0 or 500 nM CdCl2 with 0 or 1 mM NAC (2-h pretreatment). In cells treated with NAC alone, choline uptake was comparable to that in control cells (P > 0.30 vs. No Cd/No NAC; Fig. 3A). In cells exposed to 500 nM cadmium choline uptake increased by 75% (P < 0.001 vs. No Cd/No NAC), but in cadmium-exposed cells treated with NAC, uptake was comparable to that in controls (P > 0.28 vs. No Cd/No NAC). The thiol compound might have attenuated effects of cadmium by binding the metal, effectively reducing free cadmium concentration. Thus, we also tested the efficacy of Trolox, a water-soluble vitamin E analog and ROS scavenger (72), to abate cadmium-induced stimulation of choline uptake. Following 12-h exposure to 500 nM CdCl2 in the absence and presence of 100 μM Trolox (no pretreatment), 30-min apical choline uptake (10 μM [3H]choline ± 750 μM HC-3) was assayed in cadmium-free buffer without Trolox (triplicate measures, two separate culture preparations). Cadmium exposure stimulated choline uptake by 70% [6,233 ± 257 (SD) pmol/mg protein vs. 3,665 ± 151 (SD) pmol/mg protein, No Cd/No Trolox]. Trolox diminished stimulation of choline uptake in cadmium-exposed cells [4,004 ± 153 (SD) pmol/mg protein], but alone, Trolox was without effect [3,757 ± 115 (SD) pmol/mg protein]. In contrast to antioxidant treatment, BSO treatment augmented stimulation of apical choline uptake in cadmium-exposed cells (Fig. 3B). Consistent with the initial experiments, 12-h exposure to 100 nM cadmium did not alter choline uptake, and exposures to 250 nM and 500 nM cadmium increased uptake by 15% and 40% (P < 0.05 vs. No Cd/No BSO). BSO alone (100 μM, 12-h preincubation plus 12-h treatment) did not alter choline uptake (P > 0.61 vs. No Cd/No BSO). However, in the presence BSO exposures to 100, 250, and 500 nM, cadmium stimulated choline uptake by 40%, 65%, and 75%. Thus, in cadmium-exposed cells, attenuation of oxidative stress suppressed stimulation of apical choline uptake, and exacerbation of oxidative stress augmented stimulation.
Fig. 3.
Apical uptake of [3H]choline in cadmium-exposed cultured choroid plexus epithelial cells treated with N-acetylcysteine (NAC) or l-buthionine sulfoximine (BSO). A: confluent monolayers grown on impermeable supports were pretreated in serum-free for 12 h with 1 mM NAC before 12-h exposure to 0 or 500 nM CdCl2 with 1 mM NAC; in parallel, representative cells were pretreated in serum-free medium for 12 h without NAC before 12-h exposure to 0 or 500 nM CdCl2. B: cells were pretreated in serum-free for 12 h with 100 μM BSO before 12-h exposure to 0, 250 or 500 nM CdCl2 and 100 μM BSO; in parallel, representative cells were pretreated in serum-free medium for 12 h without BSO before 12-h exposure to 0, 250, or 500 nM CdCl2 without BSO. A and B: after treatment, cells were rinsed and incubated (30 min, 37°C) in artificial CSF (10 mM Tris·HEPES, pH 7.4) with 10 μM [3H]choline chloride ± 750 μM hemicholinium-3; transport buffer did not contain cadmium, NAC or BSO. [3H]Choline uptake was expressed as the percentage of mediated uptake in nontreated control cells, i.e., 24 h in serum-free medium without cadmium, NAC, or BSO. Values are expressed as means ± SE; effects of NAC and BSO were each tested in three separate culture preparations (n = 3). *Significantly different than nontreated controls (No Cd/No NAC/No BSO); P < 0.05.
Dependence of stimulation of apical choline uptake on activation of extracellular signal regulated kinase-1/2 (ERK1/2) in cadmium-exposed CPE cells.
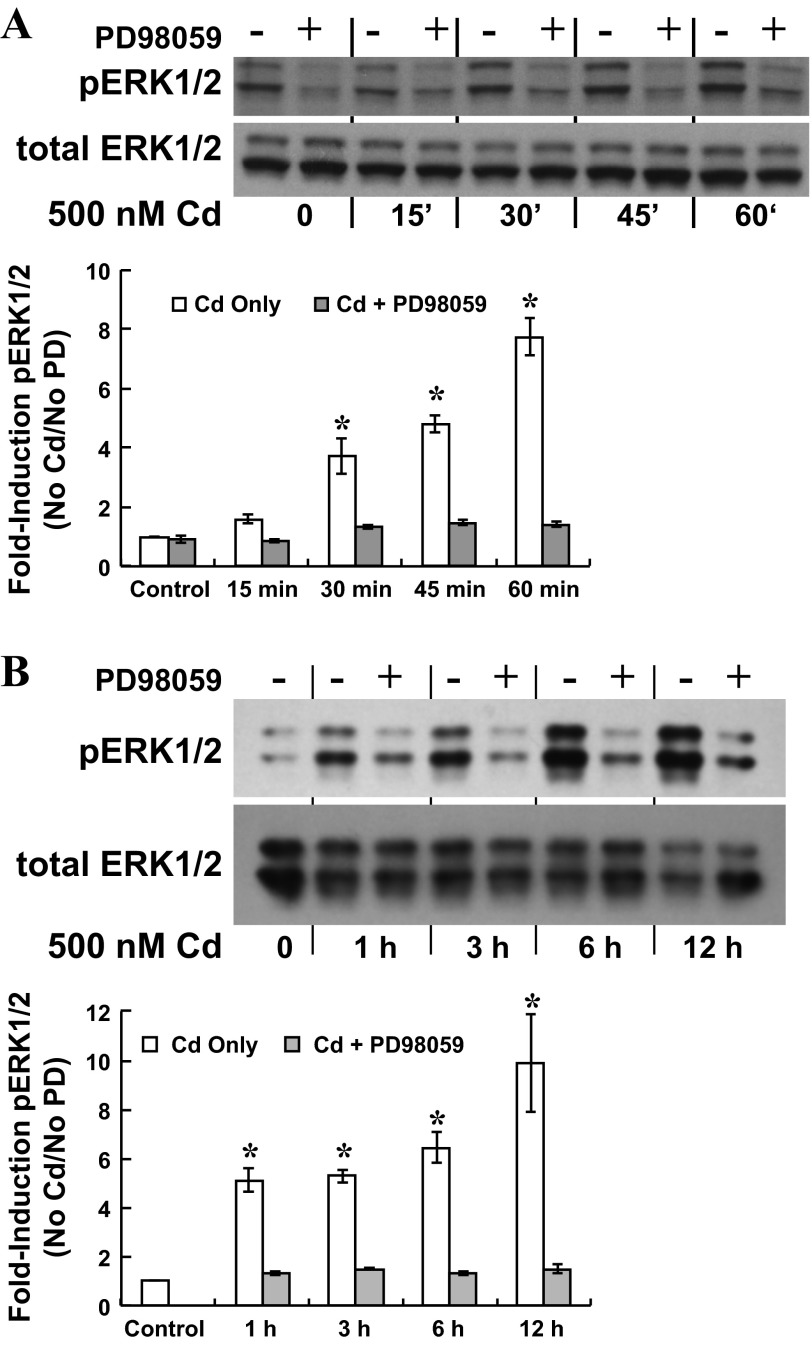
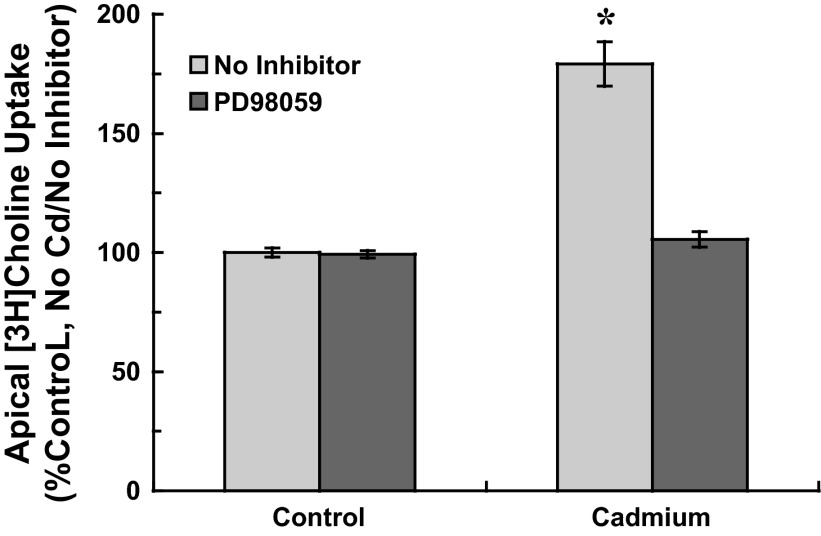
In epithelial and other cell types, ERK1/2 MAP kinase may be activated by cadmium through induction of oxidative stress (55), and organic cation transport in renal proximal tubule may be regulated through ERK1/2 signaling (49). To determine whether cadmium activated ERK1/2 in CPE cells, we analyzed phosphorylated ERK1/2 (phospho-ERK1/2) in cells treated without or with a MEK1 inhibitor PD98059. Cells were exposed to 500 nM CdCl2 with 0 or 25 μM PD98059 (2-h preincubation) for short (0–60 min) and extended (0–12 h) periods; time-dependent accumulation of phospho-ERK1/2 was measured by immunoblot analysis (Fig. 4). Phospho-ERK1/2 expression was normalized to that of total ERK1/2 (nonphosphorylated plus phosphorylated ERK1/2). For short-term cadmium exposure, PD98059-sensitive increases in phospho-ERK1/2 were detected within 15 min and remained elevated through 60 min of cadmium exposure (Fig. 4A). Similarly, at 1 h of extended cadmium exposure phospho-ERK1/2 had markedly increased and remained elevated at 12 h; PD98059 decreased ERK1/2 phosphorylation at each time point (Fig. 4B). To determine whether cadmium elicited stimulation of choline uptake through ERK1/2 signaling, we exposed cells to 500 nM CdCl2 with 0 or 25 μM PD98059 (2-h preincubation) and then assayed 30-min apical choline uptake (10 μM [3H]choline ± 750 μM HC-3) in cadmium-free buffer without PD98059 (Fig. 5). In cadmium-free cells, PD98059 treatment did not alter choline uptake (P > 0.2 vs. No Cd/No PD98059). In contrast, whereas cadmium exposure stimulated choline uptake by 80% (P < 0.003 vs. control, i.e., No Cd/No PD98059), PD98059 attenuated stimulation of uptake in cadmium-treated cells (P < 0.001 vs. Cd alone). Choline uptake in cells exposed to cadmium with PD98059 was comparable to that in controls (P > 0.3 vs. control, i.e., No Cd/No PD98059). These data indicated that stimulation of apical choline uptake was elicited through ERK1/2 activation.
Fig. 4.
Time-dependent phosphorylation of ERK1/2 in cultured choroid plexus epithelial cells exposed to cadmium with the MEK1 inhibitor, PD98059. After 12-h incubation in serum-free medium, cells were exposed to 500 nM CdCl2 with 0 or 25 μM PD98059 for 60 min or 12 h. Phosphorylated ERK1/2 (pERK1/2) was assayed by immunoblot analysis using enhanced chemiluminescent detection of immunoreactivity of primary antibody with horseradish peroxidase (HRP)-conjugated secondary antibody; membranes then were stripped and probed with primary antibody against total ERK1/2, and immunoreactivity was detected using alkaline phosphatase-conjugated secondary antibody and BCIP-NBT reagent. A: representative immunoblot and summarized data for PD98059-sensitive ERK1/2 phosphorylation during 60-min exposure to 500 nM CdCl2 ± 25 μM PD98059 (n = three separate culture preparations). *Significantly different than nontreated 60-min controls, i.e., No Cd/No PD98059; P < 0.05. B: representative immunoblot and summarized data for PD98059-sensitive ERK1/2 phosphorylation during 12-h exposure to 500 nM CdCl2 ± 25 μM PD98059 (n = 3 separate culture preparations); *Significantly different than nontreated 12-h controls (No Cd/No PD98059); P < 0.05.
Fig. 5.
Apical [3H]choline uptake in cultured choroid plexus epithelial cells exposed to cadmium in serum-free medium with the MEK1 inhibitor, PD98059. Cells were incubated in serum-free medium for 12 h; during the last 2 h, cells were treated with 0 or 25 μM PD98059. Cells then were exposed for 12-h to 0 or 500 nM CdCl2 with 0 or 25 μM PD98059. After treatment, cells were rinsed and incubated (37°C, 30 min) in artificial CSF with 10 μM [3H]choline ± 750 μM hemicholinium-3; transport buffer did not contain cadmium or PD98059. [3H]Choline uptake was expressed as a percentage of mediated uptake in control cells incubated for 24 h in serum-free medium without cadmium or PD98059. Values are expressed as means ± SE; n = 3 separate culture preparations. *Significantly different than nontreated controls (No Cd/No PD98059); P < 0.05.
Disrupted modulation of apical choline uptake in cadmium-exposed CPE cells by inhibition of ERK1/2 activation despite manipulation of oxidative stress.
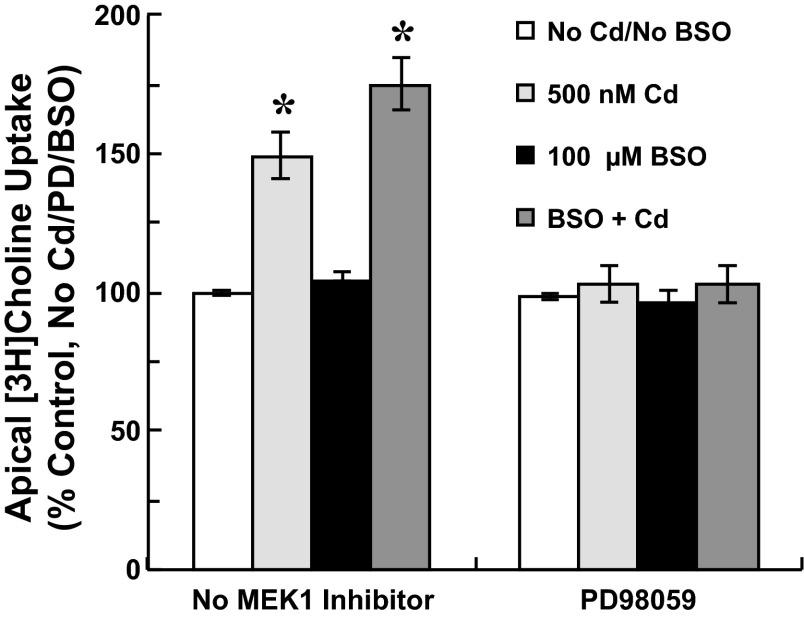
To probe the role of ERK1/2 activation in modulation of apical choline uptake in response to oxidative stress, we examined the effects of NAC and BSO on ERK1/2 activation in cadmium-exposed cells (Fig. 6). Cells were exposed for 12 h to 0–500 nM CdCl2 and treated without or with 1 mM NAC or 100 μM BSO. Twelve-hour exposure to 250 nM and 500 nM cadmium elicited sustained phosphorylation of ERK1/2. Treatment with NAC attenuated ERK1/2 phosphorylation. Treatment with antioxidant also decreased induction of Hsp70 and HO-1 proteins at each cadmium concentration. In contrast, BSO treatment increased ERK1/2 phosphorylation elicited by 250 nM and 500 nM cadmium, but also by 100 nM cadmium. Concentration-dependent induction of Hsp70 and HO-1 in response to cadmium was modestly increased when cells were treated with cadmium in the presence of BSO. Finally, to determine whether ERK1/2 activation was requisite to stimulation of choline uptake in response to oxidative stress in cadmium-exposed cells, we tested the efficacy of inhibiting ERK1/2 activation to abate stimulation of uptake when oxidative stress was exacerbated by BSO. Cells were pretreated (12 h) with 0 or 100 μM BSO before 12-h exposure to 0 or 500 nM CdCl2 with 0 or 25 μM PD98059 (2-h pretreatment). Apical choline uptake (10 μM [3H]choline ± 750 μM HC-3; 30 min) was assayed in the absence of cadmium, PD98059, and BSO (Fig. 7). Compared with control treatment, cadmium treatment increased choline uptake by 49% (P < 0.05 vs. No Cd/No BSO/No PD98059), and cadmium treatment with BSO increased uptake by 75% (P < 0.05 vs. No Cd/No BSO/No PD98059). However, PD98059 treatment attenuated stimulation of choline uptake in cadmium-exposed cells such that uptake was comparable to that in untreated controls. Likewise, choline uptake in cells exposed to cadmium with both BSO and PD98059 was comparable to uptake in nontreated controls. BSO or PD98059 treatment alone did not alter choline uptake; combined treatment with BSO and PD98059 was also without effect.
Fig. 6.
Phosphorylation of ERK1/2 and induction of stress proteins in cultured choroid plexus epithelial cells exposed for 12 h to cadmium with N-acetylcysteine (NAC) or l-buthionine sulfoximine (BSO). After 12-h incubation in serum-free medium, cells were exposed to 0, 100, 250 or 500 nM CdCl2 without or with 1 mM NAC or 100 μM BSO. Phosphorylated ERK1/2 (pERK1/2) was assayed by immunoblot analysis using enhanced chemiluminescence to visualize immunoreactivity of primary antibody with HRP-conjugated secondary antibody; membranes were then stripped and probed with primary antibody against total ERK1/2, and immunoreactivity was detected using alkaline phosphatase-conjugated secondary antibody and BCIP-NBT reagent. Immunoreactivity of Hsp70, HO-1, and β-actin with the respective primary antibody was also visualized by colorimetric detection using alkaline phosphatase-conjugated secondary antibody and BCIP/NBT reagent. Representative immunoblots (A) and summarized data (B) for phosphorylated ERK1/2, Hsp70, and HO-1 after 12-h exposure to 0, 100, 250, or 500 nM CdCl2 without or with 1 mM NAC (n = 3) or 0, 100, 250, or 500 nM CdCl2 without or with 100 μM BSO (n = 3). *Significantly different than cadmium alone (Cd/No NAC or BSO); P < 0.05.
Fig. 7.
Apical [3H]choline uptake in cultured choroid plexus epithelial cells exposed to cadmium with an inhibitor of glutathione synthesis, l-buthionine sulfoximine (BSO) and PD98059 to inhibit MEK1-mediated ERK1/2 phosphorylation. Cells plated on impermeable supports were preincubated in serum-free medium with 0 or 100 μM BSO for 12 h; during the last 2 h, representative cells were treated with 0 or 25 μM PD98059. Cells then were exposed for 12 h to 0 or 500 nM CdCl2 in the absence or presence of 100 μM BSO and 25 μM PD98059. After treatment, cells were rinsed and incubated (37°C, 30 min) in artificial CSF (10 mM Tris·HEPES, pH 7.4) with 10 μM [3H]choline ± 750 μM hemicholinium-3; transport buffer did not contain cadmium, BSO, or PD98059. [3H]Choline uptake was expressed as the percentage of mediated uptake in nontreated control cells incubated for 24 h in serum-free medium without cadmium, BSO or PD98059. Values are expressed as mean ± SE; n = 3 separate culture preparations. *Significantly different than nontreated controls (No Cd/No BSO/No PD98059); P < 0.05.
DISCUSSION
Although studies in rodents and rabbits have established that choroid plexus accumulates cadmium (53, 59, 71), how cadmium may alter solute transport in the epithelium remains elusive. These data for primary cultures of neonatal rat choroid plexus epithelial (CPE) cells indicate exposure to low concentrations of cadmium may modulate apical membrane transport of organic solute, e.g., choline, at the blood-CSF barrier. In CPE cells, transport of choline into the cell (i.e., uptake) across the apical membrane is analogous to transport of choline from CSF into the epithelial cell across the ventricular membrane and, thus, represents the initial removal of choline from the CSF compartment. At concentrations up to 1,000 nM, extracellular cadmium (CdCl2) did not directly decrease or increase apical uptake of choline. However, extended pretreatment with 50 nM to 1,000 nM cadmium increased apical choline uptake as a function of the duration of treatment and the concentration of cadmium. We elucidated modulation of choline uptake in CPE cells exposed for 12 h to cadmium (CdCl2) at concentrations not exceeding 500 nM. Cadmium-exposed cells mounted a cellular stress response, as indicated by induction of Hsp70 protein and upregulation of HO-1 protein and Mt-1 mRNA. Treatment with an antioxidant, NAC, attenuated the increase in apical choline uptake in cadmium-exposed cells. Conversely, treatment with BSO, an inhibitor of glutathione synthesis, further increased choline uptake in cadmium-exposed cells. This suggested choline uptake was modulated in response to oxidative stress. As a first approach to identify signaling pathways that mediate modulation of choline uptake, we analyzed activation of ERK1/2 MAP kinase. Cadmium exposure activated ERK1/2, but inhibition of ERK1/2 activation with PD98059 abated stimulation of choline uptake in cadmium-exposed cells. Treatment with NAC attenuated ERK1/2 activation, whereas treatment with BSO enhanced phosphorylation of the MAP kinase. This was consistent with activation of ERK1/2 in response to oxidative stress. PD98059 treatment also abated stimulation of apical choline uptake in cells exposed to both cadmium and BSO. Thus, despite the persistent state of oxidative stress, inhibition of ERK1/2 activation attenuated modulation of choline transport in cadmium-exposed cells. These data indicated in CPE cells stimulation of apical choline transport was elicited in response to oxidative stress and mediated through ERK1/2 signaling.
The intact choroid plexus sequesters contaminant heavy metals and excess nutritive minerals, thereby minimizing accumulation of these potentially neurotoxic agents in the central nervous system (20, 69, 71). The epithelium most likely accumulates minerals and heavy metals across both the basolateral and apical membranes from blood and CSF, respectively. Rodent choroid plexus expresses transporters for nutritive minerals, including several ZnT (SLC30A) and ZIP (Zrt-/Irt-related proteins, SLC39A) zinc transporters (6, 12, 16, 66). The divalent metal transporter-1, iron-regulated transporter, and transferrin receptor that facilitate iron uptake and possibly manganese transport are expressed (9, 17, 41), as are copper transporters, Ctr1 and ATP7A (14). Transporters that mediate uptake and sequestration of specific contaminant metals in choroid plexus have yet to be identified. However, in renal tubule and other epithelia, zinc and iron transporters can mediate cellular accumulation of heavy metals, such as cadmium, lead, and mercury (8, 21). Similarly, transport systems for essential minerals could also mediate transport and sequestration of heavy metals in the choroid plexus. We confirmed CPE cells plated on solid supports accumulated cadmium across the apical membrane. We propose iron and zinc transporters might mediate apical uptake of Cd2+ in CPE cells plated on solid supports. DMT1, which can transport cadmium (21) and is expressed in the apical membrane of choroid plexus (64), could mediate apical Cd2+ uptake in CPE cells. The zinc importers (ZIPs) can also mediate cellular uptake of cadmium (8, 21), and ZIP6, expressed in the apical membrane of choroid plexus (16), could mediate cellular uptake of cadmium across the apical membrane of CPE cells.
Stimulation of apical choline uptake in cadmium-exposed CPE cells was not expected, on the basis of prior reports that cadmium elicited cellular injury in choroid plexus and can inhibit membrane solute transport. As cited earlier, chronic in vivo cadmium exposure elicited changes in mouse choroid plexus ultrastructure, such as shortening and loss of microvilli and formation of intracellular vacuoles (59). At concentrations or exposure periods exceeding those we tested, cadmium can directly inhibit ion fluxes and organic cation transport. For example, in isolated rat choroid plexus and cultured porcine choroid plexus cells 30–1,000 μM extracellular cadmium incrementally reduced apical inward-rectifying chloride conductance (24, 25). In human erythrocytes, 100–300 μM cadmium inhibited 3-h uptake of 10 μM choline (28), and in rats, 4 mM cadmium inhibited influx of 0.013 μM choline at the intact blood-brain barrier (36). Organic cation transporter-2 (OCT2) mediates apical choline transport in rat choroid plexus (51), and in Chinese hamster ovary (CHO) cells transfected with rabbit OCT2 (rbOCT2), 10 nM tetraethylammonium (TEA) transport was cis-inhibited by cadmium with an IC50 of 207 μM (50). However, in CPE cells 50–1,000 nM cadmium did not directly decrease or increase apical choline transport (Table 1), but in a time- and concentration-dependent manner, extended cadmium exposure stimulated choline transport (Fig. 1). Also, the duration and concentration of cadmium exposure used subsequently to characterize modulation of apical choline uptake, i.e., 12-h treatment with 500 nM cadmium, did not markedly increase LDH release compared with that from control cells. This indicated the in vitro dose of cadmium was not overtly cytotoxic. We did not test the potency of cadmium beyond 1 μM to cis-inhibit apical uptake of 10 μM choline in CPE cells, as our objective was to elucidate modulation of solute transport by nonlethal exposure to cadmium.
At high doses, cadmium and other heavy metals can be acutely toxic and progressively elicit cell injury, cell death, and organ failure, whereas at low doses the same metals may modulate specific mechanisms such as membrane transport without marked cellular or systemic toxicity. At nanomolar to micromolar concentrations, cadmium may directly or secondarily impair carrier- and channel-mediated fluxes of solute, e.g., Na,K-ATPase activity and glucose transport via SLGT [reviewed extensively by Van Kerkhove et al. (60)]. Nevertheless, stimulation of apical choline uptake in CPE cells exposed to submicromolar cadmium is not altogether exceptional. In Caco-2 intestinal cells, 24-h exposure to 1 μM cadmium increased multidrug resistance transporter (MDR) expression and MDR-mediated calcein-M efflux (23). Similarly, in isolated killifish renal tubules 24-h exposure to 500 nM cadmium increased multidrug-related protein-2 (MRP2) expression and MRP2-mediated fluorescein-methotrexate secretion (54). Inorganic mercury and arsenic may also upregulate solute transport. Extended exposure (24–48 h) to 1 μM mercuric chloride increased GLUT1 protein and GLUT1-mediated glucose uptake in adipocytes (4). Arsenite may modulate multidrug resistance, as indicated by upregulation of MRP1, MRP2, and MDR in TRL125 liver cells exposed to 125–500 nM arsenite for 24 days (35). In killifish, 14-day exposure to 1,000 ppb arsenite (an environmentally relevant concentration) induced renal tubular MRP2 expression and MRP2-mediated fluorescein-methotrexate secretion (40). Collectively, data for CPE cells and other epithelial tissues suggest low doses of metals (or metalloids) that might not induce marked toxicity or cell injury may still modulate membrane transport, a function critical to an epithelium's biology and its role in the animal's physiology.
Studies in rabbits had indicated choline accumulation by choroid plexus was regulated by sympathetic innervation and hormones, such as estrogen, progesterone, and thyroid hormone (33, 34). These data for rat CPE cells suggest apical choline transport may be upregulated under conditions of oxidative stress induced by cadmium. Cadmium-exposed cells mounted a cellular stress response, as indicated by induction of Hsp70 (Fig. 2) (30). As also characteristic of in vitro and in vivo cadmium exposures (18, 58), expression of the antioxidant enzyme HO-1 and gene expression of the metal-binding protein Mt-1 were induced (Fig. 2). Metallothionein is upregulated by heavy metals but also oxidative stress (13), and its induction had been observed in intact choroid plexus of cadmium-exposed rodents (15, 45, 71). A precursor to the free radical scavenger glutathione, NAC abated stimulation of apical choline uptake in cadmium-exposed cells (Fig. 3), as did the vitamin E analog, Trolox. In contrast, BSO, an inhibitor of glutathione synthesis, enhanced stimulation of choline uptake in cadmium-exposed cells (Fig. 3). These data indicated oxidative stress was a signaling event in stimulation of apical choline uptake in cultured choroid plexus epithelial cells.
In renal proximal tubule and liver oxidative stress elicited by cadmium or other agents can upregulate efflux of solute via multidrug transporters. This may serve to remove potentially toxic metabolites from the cell, reducing risk of cell injury or death. In Wistar-Kyoto proximal tubule (WKPT) cells, 3-day exposure to 5 μM cadmium induced ROS accumulation and increased MDR1-mediated calcein efflux. However, NAC attenuated ROS accumulation and abated increases in MDR1 expression and activity, indicating ROS accumulation was a signaling factor in modulation of transport. In addition, inhibiting MDR1 activity with PSC833 increased cadmium-induced apoptosis. Thus, the authors proposed upregulation of MDR1-facilitated efflux of cadmium complexes and ceramides, which might otherwise induce apoptosis (56, 57). Similarly, in mouse liver, inducing oxidative stress by acetaminophen overdose (3) or genetic impairment of glutathione synthesis genes (38) can increase MRP2, MRP3, or MRP4 expression, possibly regulated by binding of NRF2 to the antioxidant response element in promoter regions of the respective mrp genes (38). Increased MRP transporter activity may mediate removal of toxic metabolites of xenobiotics or byproducts of reactive oxygen or nitrogen species and, thus, attenuate cellular accumulation of cytotoxic compounds and facilitate recovery from cellular injury (3). As shown in immortalized Z310 choroid plexus, epithelial cells induction of oxidative stress with H2O2 increased cystine transport; it was suggested that increased uptake of cystine, a precursor to glutathione, might support increased synthesis of glutathione (67). However, it remains unclear whether increased cellular uptake of choline or other organic cations by a common apical transporter would be cytoprotective. This was beyond the scope of the present study, but it should be addressed in future studies to better understand how the blood-CSF barrier adapts to physicochemical stressors.
In CPE cells, cadmium activated ERK1/2 MAP kinase, but inhibition of ERK1/2 activation attenuated stimulation of apical choline uptake (Figs. 4 and 5), indicating ERK1/2 signaling regulated modulation of choline uptake. This was consistent with reports that cadmium elicited ERK1/2 activation in other epithelial cells and that ERK1/2 signaling regulated organic cation transport in renal proximal tubule (49, 55). In HEK293 cells transfected with human OCT2, 1 μM U-0126 (which inhibits MEK1) did not alter 4-[4-(dimethylamino)styryl]-N-methyl-pyridinium transport (11). However, 10 μM U-0126 inhibited basal transport of TEA in rbOCT2-transfected CHO cells and disrupted EGF-induced stimulation of basolateral TEA uptake in isolated rabbit proximal tubule (49). In our study, the MEK1 inhibitor PD98059 did not reduce apical choline uptake in untreated cells, but abrogated stimulation of choline uptake in cadmium-exposed cells. In rat hepatocytes, NAC treatment can disrupt ERK1/2 activation elicited by cadmium (68). Others have cited cadmium may bind to high-affinity SH-groups in NAC or the glutathione generated from NAC; thus, NAC may indirectly attenuate oxidative stress by chelating cadmium (55). Nevertheless, in cadmium-exposed CPE cells, NAC attenuated ERK1/2 activation and stimulation of apical choline uptake, and BSO augmented both ERK1/2 activation and stimulation of choline uptake. Furthermore, the MEK1 inhibitor PD98059 impeded stimulation of choline uptake in cadmium-exposed cells, and its efficacy persisted during combined exposure to cadmium and BSO (Fig. 7). These data indicate that cadmium activated ERK1/2 through induction of oxidative stress, and stimulation of apical choline uptake in response to oxidative stress is mediated through ERK1/2.
It is unclear whether ERK1/2 might also regulate cytoprotective mechanisms to promote cell survival and maintain functional integrity in CPE cells subjected to a low-dose cadmium exposure. Cadmium elicited sustained activation of ERK1/2 through 12 h (Fig. 4); however, inhibiting ERK1/2 activation abated stimulation of choline transport and increased cytotoxicity (e.g., increased LDH release). Generally, ERK1/2, JNK, and p38 MAP kinases are activated within minutes of initiation of the stimulus, and activation is short-lived. Conventional thought holds that transient ERK1/2 activation signals cell proliferation, whereas sustained ERK1/2 activation in response to physical, chemical, or pathological stressors is associated with apoptosis (39, 47, 55). However, the nature of the cellular response and which MAP kinase is activated by cadmium can vary with cadmium concentration, exposure duration, and cell or tissue type. Sustained ERK1/2 activation in response to oxidative stress can mediate cell survival in cadmium-treated cells. In immortalized human B cells, 24-h exposure to 10 μM cadmium induced oxidative stress and HO-1 expression and elicited sustained activation of ERK1/2; inhibition of ERK1/2 with PD98059 attenuated HO-1 expression (44). In WKPT cells, 6-h exposure to 25 μM cadmium elicited ROS-dependent activation of ERK1/2 that, in turn, mediated bestrophin-3-dependent prevention of endoplasmic reticulum stress (32). We have not established the role of ERK1/2 in upregulation of Hsp70, metallothionein, or HO-1 in cadmium-treated CPE cells or determined whether p38, JNK, or other signaling axes are activated. Identifying the signaling pathways that might integrate cytoprotective mechanisms with modulation of membrane transport would lend further insight into how the choroid plexus epithelium adapts to physicochemical stressors.
In summary, these data for primary cultures of choroid plexus epithelial cells indicate cellular uptake of choline across the apical membrane is stimulated by cadmium at relatively low concentrations. More specifically, apical choline transport is stimulated through ERK1/2 signaling activated in response to oxidative stress induced by cadmium. Further studies are needed to determine whether other organic and inorganic transport systems in choroid plexus are modulated in response to low doses of cadmium or other heavy metals and to elucidate how ERK1/2 and other signaling factors might possibly integrate upregulation of cytoprotective mechanisms with modulation of solute transport.
Perspectives and Significance
These findings suggest that while the choroid plexus sequesters heavy metals and excess nutritive minerals and, thereby, minimizes accumulation of these potentially toxic agents in the brain, the cell biology and physiology of the epithelium may be perturbed, as it adapts to cellular stress induced by metals, such as cadmium. Membrane transport of organic and inorganic solutes in the choroid plexus is central to secretion of CSF and selective exchange of bioactive compounds and macromolecules at this interface of blood and the extracellular fluid compartment of the brain. Thus, modulation of transport under mild or severe stress conditions could, in turn, disrupt fluid/electrolyte balance and alter nutrient availability and clearance of xenobiotic and metabolic wastes in the brain. Choline, the model organic cation used in these studies, is the precursor to the neurotransmitter acetylcholine. The intact choroid plexus actively clears choline from CSF into blood across the blood-CSF barrier (37). Apical choline uptake in CPE cells is analogous to the initial removal of choline from CSF across the ventricular membrane into the intact choroid plexus epithelium. Hypothetically, if in vivo low-dose exposure to cadmium stimulated apical choline transport in intact choroid plexus, this could increase choline removal from CSF and potentially reduce choline availability in the brain. However, it should be clarified that a caveat to using CPE cells plated on impermeable substrata permits discrete, albeit limited, access to the apical pole of the epithelium. Thus, direct assay of solute transport was limited to the apical pole, and cadmium accumulates across only the apical membrane. Therefore, we cannot predict whether cadmium exposure would also upregulate basolateral transport of choline, such that net transepithelial absorption and clearance of choline from CSF to blood would increase. If the basolateral membrane were also exposed to cadmium, as would be the case in vivo or in CPE cells on permeable supports, then at a given dose of cadmium, total cellular accumulation of metal could be greater, and the cellular responses could be enhanced. Nevertheless, CPE cells plated on impermeable supports accumulated cadmium and, thus, they provide insight into the possible modulation of membrane transport and induction of cellular stress elicited by sequestration of a model contaminant metal. These data, herein, suggest that induction of oxidative stress elicited by heavy metal exposure or other stressors, such as hyperthermia, hyperosmotic shock, and ischemia could be an initiating or earlier event in signaling for modulation of transport of choline or other bioactive compounds. ERK1/2 and other signaling axes may be activated in a sustained manner in response to oxidative stress and mediate changes in transport and cytoprotective mechanisms. How rapidly choroid plexus adapts to nonlethal stress and whether the barrier epithelium retains the capacity to respond to subsequent physiological or stress stimuli is unclear.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.R.A.V. conception and design of research; A.R.A.V. and R.K.Y. performed experiments; A.R.A.V. and R.K.Y. analyzed data; A.R.A.V. and R.K.Y. interpreted results of experiments; A.R.A.V. and R.K.Y. prepared figures; A.R.A.V. and R.K.Y. drafted manuscript; A.R.A.V. and R.K.Y. edited and revised manuscript; A.R.A.V. and R.K.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by research awards from the National Science Foundation (IOS1052654) and a National Institutes of Health-National Institute of Environmental Health Sciences Grant (ES-10439).
REFERENCES
- 1.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25: 5–23, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances, and Disease Registry Agency for Toxic Substances, and Disease Registry (ATSDR). Toxicological Profile for Cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, 2012 [Google Scholar]
- 3.Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol 226: 74–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes DM, Kircher EA. Effects of mercuric chloride on glucose transport in 3T3-L1 adipocytes. Toxicol In Vitro 19: 207–214, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Behl M, Zhang Y, Shi Y, Cheng J, Du Y, Zheng W. Lead-induced accumulation of β-amyloid in the choroid plexus: role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology 31: 524–532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belloni-Olivi L, Marshall C, Laal B, Andrews GK, Bressler J. Localization of zip1 and zip4 mRNA in the adult rat brain. J Neurosci Res 87: 3221–3230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake MJ, Nowak TS, Jr, Holbrook NJ. In vivo hyperthermia induces expression of HSP70 mRNA in brain regions controlling the neuroendocrine response to stress. Brain Res Mol Brain Res 8: 89–92, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204: 274–308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 66: 1198–1207, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346: 171–179, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Cetinkaya I, Ciarimboli G, Yalcinkaya G, Mehrens T, Velic A, Hirsch JR, Gorboulev V, Koepsell H, Schlatter E. Regulation of human organic cation transporter hOCT2 by PKA, PI3K, and calmodulin-dependent kinases. Am J Physiol Renal Physiol 284: F293–F302, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chi ZH, Wang X, Wang ZY, Gao HL, Dahlstrom A, Huang L. Zinc transporter 7 is located in the cis-Golgi apparatus of mouse choroid epithelial cells. Neuroreport 17: 1807–1811, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Chiaverini N, De Ley M. Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res 44: 605–613, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res 1248: 14–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhuri S, Liu WL, Berman NE, Klaassen CD. Cadmium accumulation and metallothionein expression in brain of mice at different stages of development. Toxicol Lett 84: 127–133, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Chowanadisai W, Kelleher SL, Lonnerdal B. Zinc deficiency is associated with increased brain zinc import and LIV-1 expression and decreased ZnT-1 expression in neonatal rats. J Nutr 135: 1002–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology 24: 3–13, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals 23: 927–940, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Ennis SR, Keep RF. The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab 26: 675–683, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Friedheim E, Corvi C, Graziano J, Donnelli T, Breslin D. Choroid plexus as a protective sink for heavy metals? Lancet 1: 981–982, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Fujishiro H, Yano Y, Takada Y, Tanihara M, Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 4: 700–708, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Gu H, Wei X, Monnot AD, Fontanilla CV, Behl M, Farlow MR, Zheng W, Du Y. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci Lett 490: 16–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huynh-Delerme C, Huet H, Noel L, Frigieri A, Kolf-Clauw M. Increased functional expression of P-glycoprotein in Caco-2 TC7 cells exposed long-term to cadmium. Toxicol In Vitro 19: 439–447, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kajita H, Omori K, Matsuda H. The chloride channel ClC-2 contributes to the inwardly rectifying Cl− conductance in cultured porcine choroid plexus epithelial cells. J Physiol 523: 313–324, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajita H, Whitwell C, Brown PD. Properties of the inward-rectifying Cl− channel in rat choroid plexus: regulation by intracellular messengers and inhibition by divalent cations. Pflügers Arch 440: 933–940, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kang YJ, Enger MD. Cadmium inhibits EGF-induced DNA synthesis but increases cellular glutathione levels in NRK-49F cells. Toxicology 66: 325–333, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Keep RF, Xiang J. Choroid plexus taurine transport. Brain Res 715: 17–24, 1996 [DOI] [PubMed] [Google Scholar]
- 28.King RG, Sharp JA, Boura AL. The effects of Al3+, Cd2+, and Mn2+ on human erythrocyte choline transport. Biochem Pharmacol 32: 3611–3617, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Krueger-Naug AM, Hopkins DA, Armstrong JN, Plumier JC, Currie RW. Hyperthermic induction of the 27-kDa heat shock protein (Hsp27) in neuroglia and neurons of the rat central nervous system. J Comp Neurol 428: 495–510, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67: 225–257, 2005 [DOI] [PubMed] [Google Scholar]
- 31.L'Hoste S, Chargui A, Belfodil R, Duranton C, Rubera I, Mograbi B, Poujeol C, Tauc M, Poujeol P. CFTR mediates cadmium-induced apoptosis through modulation of ROS level in mouse proximal tubule cells. Free Radic Biol Med 46: 1017–1031, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Lee WK, Chakraborty PK, Roussa E, Wolff NA, Thevenod F. ERK1/2-dependent bestrophin-3 expression prevents ER-stress-induced cell death in renal epithelial cells by reducing CHOP. Biochim Biophys Acta 1823: 1864–1876, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Lindvall M, Owman C, Winbladh B. Sympathetic influence on transport functions in the choroid plexus of rabbit and rat. Brain Res 223: 160–164, 1981 [DOI] [PubMed] [Google Scholar]
- 34.Lindvall-Axelsson M, Owman C. Actions of sex steroids and corticosteroids on rabbit choroid plexus as shown by changes in transport capacity and rate of cerebrospinal fluid formation. Neurol Res 12: 181–186, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol 60: 302–309, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Lockman PR, Roder KE, Allen DD. Inhibition of the rat blood-brain barrier choline transporter by manganese chloride. J Neurochem 79: 588–594, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Loffelholz K, Klein J, Koppen A. Choline, a precursor of acetylcholine and phospholipids in the brain. Prog Brain Res 98: 197–200, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, Dalton TP, Yamamoto M, Klaassen CD. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology 46: 1597–1610, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Martin P, Pognonec P. ERK and cell death: cadmium toxicity, sustained ERK activation and cell death. FEBS J 277: 39–46, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Miller DS, Shaw JR, Stanton CR, Barnaby R, Karlson KH, Hamilton JW, Stanton BA. MRP2 and acquired tolerance to inorganic arsenic in the kidney of killifish (Fundulus heteroclitus). Toxicol Sci 97: 103–110, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Moos T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol 375: 675–692, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Murphy VA, Johanson CE. Na+-H+ exchange in choroid plexus and CSF in acute metabolic acidosis or alkalosis. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1528–F1537, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura M, Yasutake A, Fujimura M, Hachiya N, Marumoto M. Effect of methylmercury administration on choroid plexus function in rats. Arch Toxicol 85: 911–918, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Nemmiche S, Chabane-Sari D, Kadri M, Guiraud P. Cadmium-induced apoptosis in the BJAB human B cell line: Involvement of PKC/ERK1/2/JNK signaling pathways in HO-1 expression. Toxicology 300: 103–111, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Nishimura N, Nishimura H, Ghaffar A, Tohyama C. Localization of metallothionein in the brain of rat and mouse. J Histochem Cytochem 40: 309–315, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Cur Top Dev Biol 71: 1–52, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118: 182–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soodvilai S, Chatsudthipong A, Chatsudthipong V. Role of MAPK and PKA in regulation of rbOCT2-mediated renal organic cation transport. Am J Physiol Renal Physiol 293: F21–F27, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Soodvilai S, Nantavishit J, Muanprasat C, Chatsudthipong V. Renal organic cation transporters mediated cadmium-induced nephrotoxicity. Toxicol Lett 204: 38–42, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Sweet DH, Miller DS, Pritchard JB. Ventricular choline transport: a role for organic cation transporter 2 expressed in choroid plexus. J Biol Chem 276: 41611–41619, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Takeda A, Akiyama T, Sawashita J, Okada S. Brain uptake of trace metals, zinc and manganese, in rats. Brain Res 640: 341–344, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Takeda A, Takefuta S, Ijiro H, Okada S, Oku N. 109Cd transport in rat brain. Brain Res Bull 49: 453–457, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Terlouw SA, Graeff C, Smeets PH, Fricker G, Russel FG, Masereeuw R, Miller DS. Short- and long-term influences of heavy metals on anionic drug efflux from renal proximal tubule. J Pharmacol Exp Ther 301: 578–585, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Thevenod F. Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol 238: 221–239, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Thevenod F, Friedmann JM. Cadmium-mediated oxidative stress in kidney proximal tubule cells induces degradation of Na+/K+-ATPase through proteasomal and endo-/lysosomal proteolytic pathways. FASEB J 13: 1751–1761, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Thevenod F, Friedmann JM, Katsen AD, Hauser IA. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-κB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem 275: 1887–1896, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem 12: 1161–1208, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Valois AA, Webster WS. The choroid plexus as a target site for cadmium toxicity following chronic exposure in the adult mouse: an ultrastructural study. Toxicology 55: 193–205, 1989 [DOI] [PubMed] [Google Scholar]
- 60.Van Kerkhove E, Pennemans V, Swennen Q. Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals 23: 823–855, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Villalobos AR, Parmelee JT, Pritchard JB. Functional characterization of choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther 282: 1109–1116, 1997 [PubMed] [Google Scholar]
- 62.Villalobos AR, Parmelee JT, Renfro JL. Choline uptake across the ventricular membrane of neonate rat choroid plexus. Am J Physiol Cell Physiol 276: C1288–C1296, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Villalobos AR, Renfro JL. Trimethylamine oxide suppresses stress-induced alteration of organic anion transport in choroid plexus. J Exp Biol 210: 541–552, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Li GJ, Zheng W. Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: effect of manganese exposure. Exp Biol Med (Maywood) 233: 1561–1571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36: 1434–1443, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Wang ZY, Stoltenberg M, Jo SM, Huang L, Larsen A, Dahlstrom A, Danscher G. Dynamic zinc pools in mouse choroid plexus. Neuroreport 15: 1801–1804, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Xiang J, Alesi GN, Zhou N, Keep RF. Protective effects of isothiocyanates on blood-CSF barrier disruption induced by oxidative stress. Am J Physiol Regul Integr Comp Physiol 303: R1–R7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yiran Z, Chenyang J, Jiajing W, Yan Y, Jianhong G, Jianchun B, Xuezhong L, Zongping L. Oxidative stress and mitogen-activated protein kinase pathways involved in cadmium-induced BRL 3A cell apoptosis. Oxid Med Cell Longev 2013: 516051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng W. Toxicology of choroid plexus: special reference to metal-induced neurotoxicities. Microsc Res Tech 52: 89–103, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng W, Deane R, Redzic Z, Preston JE, Segal MB. Transport of l-[125I]thyroxine by in situ perfused ovine choroid plexus: inhibition by lead exposure. J Toxicol Environ Health 66: 435–451, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng W, Perry DF, Nelson DL, Aposhian HV. Choroid plexus protects cerebrospinal fluid against toxic metals. FASEB J 5: 2188–2193, 1991 [DOI] [PubMed] [Google Scholar]
- 72.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]