Fig. 6.
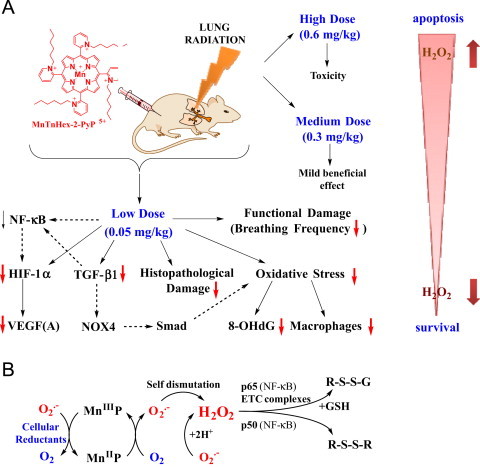
Radioprotective, therapeutic effects of MnTnHex-2-PyP5+ vs its toxicity is dose-dependent. (A) The effects reported in this work. The substantial amount of literature data strongly suggest that there is a cross-talk between HIF-1α, VEGF(A) TGF-β1 and NF-kB and likely involve the effects on NADPH oxidase isoform NOX4 [16] and Smads proteins [49], [50], [51], [52]. Effects of MnP on HIF-1α, VEGF and NOX-4 have been reported [16], [53], [54], [55]. Based on our most recent advancement in cell biology and aqueous chemistry data we believe that the in vivo mechanism of MnP is related to its redox cycling with cellular reductants and/or reactive species and oxygen as shown in Scheme B which gives rise to H2O2. The peroxide is in turn used by MnP to oxidize [55], [56] or glutathionylate [43], [45] the redox-sensitive cysteines of p50 and p65 subunits of NF-κB. Such modifications imparted by MnP suppress the transcription of NF-κB, perpetuating thus the inflammation. The glutathionylation of redox sensitive complexes I and III of mitochondrial electron transport chain and therefore (i) increase in O2− and its progeny production and (ii) decrease in cellular energy production may impact the HIF-1α, VEGF(A) and TGF-β1 pathways and in turn the lung damage [43]. If the intracellular levels of MnP and/or peroxide are high, the magnitude of NF-κB oxidation may be excessive and apoptosis may predominate. Such scenario likely occurred at ≥0.3 mg/kg/day of MnTnHex-2-PyP5+[1], [2], [39], [40], [42], [56], [57], [58], [59], [60]. In addition to the impact of MnP on pathways addressed in this work (red arrows), the Scheme also shows the pathways demonstrated previously to be involved in the actions of MnPs. It also includes those pathways which have not yet been explored in pulmonary radioprotection but are likely involved such as (Smads proteins).
