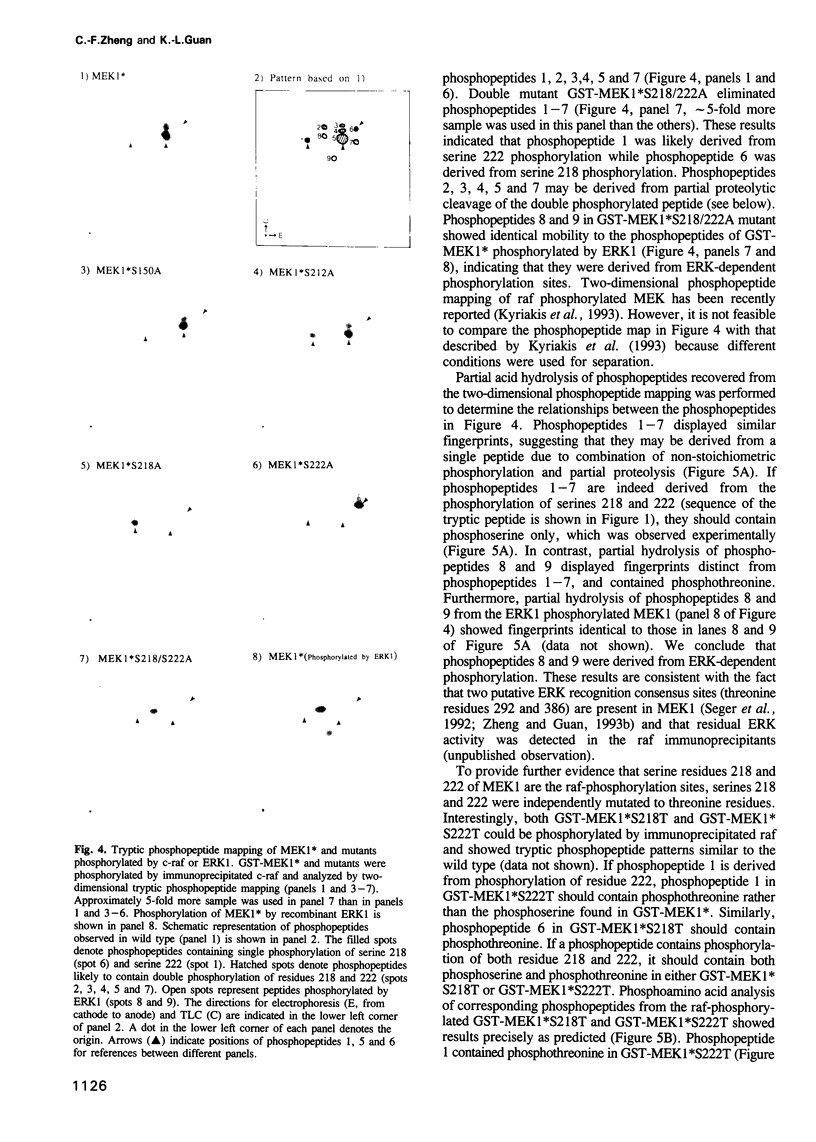
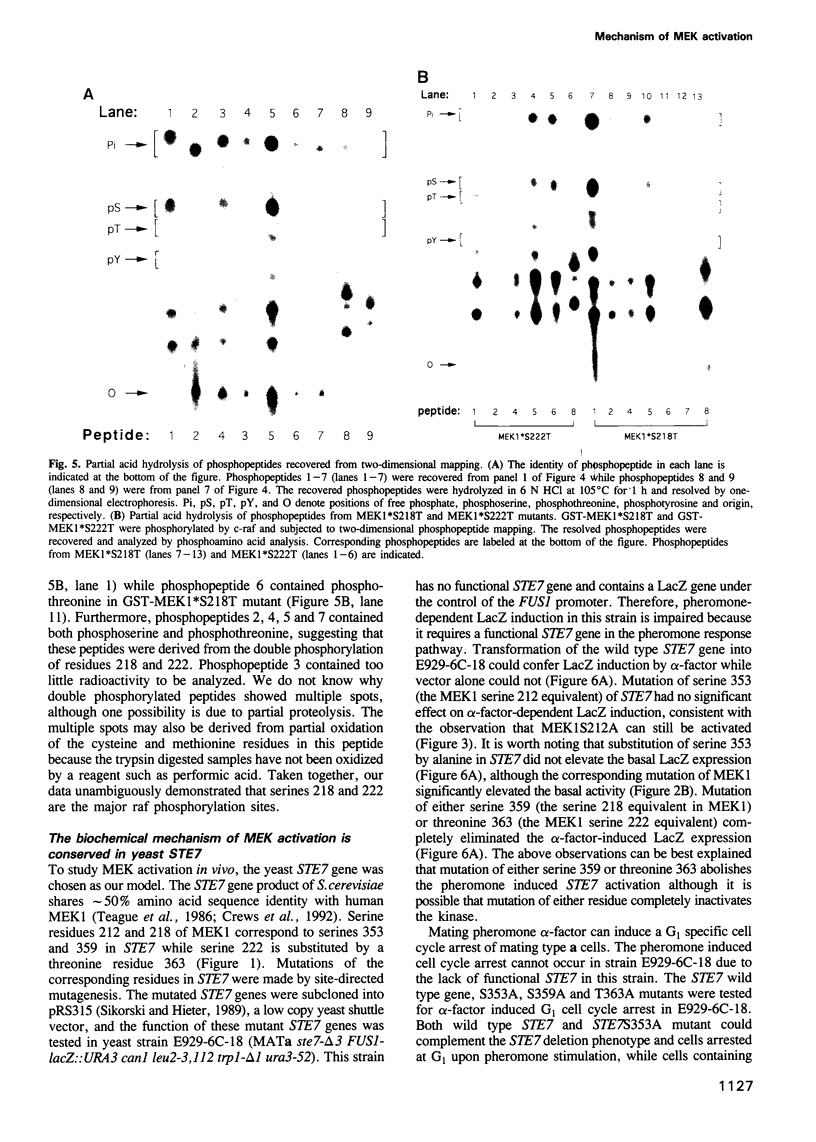
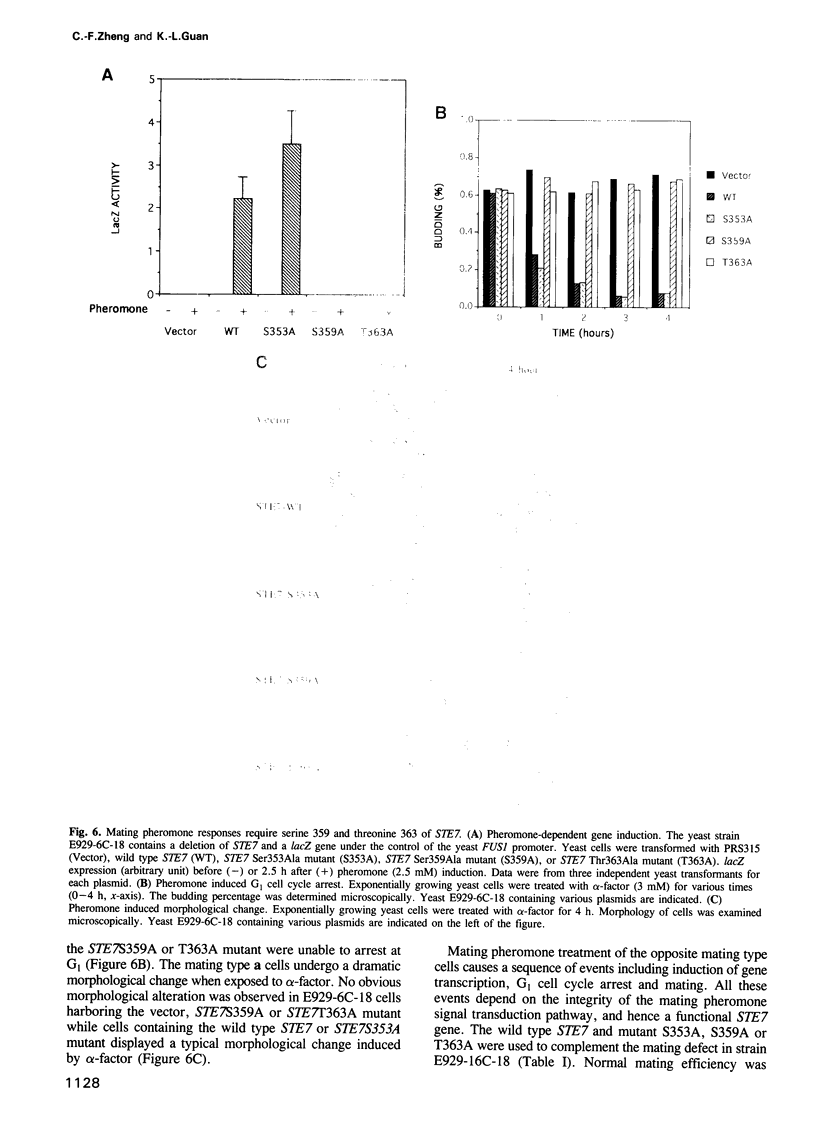
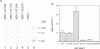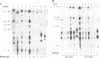Abstract
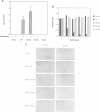
MEK is a family of dual specific protein kinases which activate the extracellular signal-regulated kinases by phosphorylation of threonine and tyrosine residues. MEK itself is activated via serine phosphorylation by upstream activator kinases, including c-raf, mos and MEK kinase. Here, we report the activation phosphorylation sites of human MEK1 and yeast STE7 kinase as determined by a combination of biochemical and genetic approaches. In human MEK1, substitution of either serine residue 218 or 222 with alanine completely abolished its activation by epidermal growth factor-stimulated Swiss 3T3 cell lysates or immunoprecipitated c-raf, suggesting that both serine residues are required for MEK1 activation. Phosphopeptide analysis demonstrated that serine residues 218 and 222 of human MEK1 are the primary sites for phosphorylation by c-raf. These two serine residues are highly conserved in all members of the MEK family, including the yeast STE7 gene product, a MEK homolog in the yeast mating pheromone response pathway. Mutation of the corresponding residues in STE7 completely abolished the biological functions of this gene. These data demonstrate that MEK is activated by phosphorylation of two adjacent serine/threonine residues and this activation mechanism is conserved in the MEK family kinases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Campbell J. S., Seger R., Jensen A. L., Graves L. M., Krebs E. G. Metabolic labeling of mitogen-activated protein kinase kinase in A431 cells demonstrates phosphorylation on serine and threonine residues. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5143–5147. doi: 10.1073/pnas.90.11.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Krebs E. G. The mitogen-activated protein kinase activator. Curr Opin Cell Biol. 1992 Dec;4(6):992–999. doi: 10.1016/0955-0674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Alessi D. R., Smythe C., Keyse S. M. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993 Jul;8(7):2015–2020. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Ashworth A., Nakielny S., Cohen P., Marshall C. The amino acid sequence of a mammalian MAP kinase kinase. Oncogene. 1992 Dec;7(12):2555–2556. [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski G., Polazzi J. O. Complete nucleotide sequence of a gene conferring polymyxin B resistance on yeast: similarity of the predicted polypeptide to protein kinases. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5848–5852. doi: 10.1073/pnas.84.16.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Charles C. H., Sun H., Lau L. F., Tonks N. K. The growth factor-inducible immediate-early gene 3CH134 encodes a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5292–5296. doi: 10.1073/pnas.90.11.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Kunisawa R., Thorner J. A putative protein kinase overcomes pheromone-induced arrest of cell cycling in S. cerevisiae. Cell. 1989 Sep 22;58(6):1107–1119. doi: 10.1016/0092-8674(89)90509-6. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Erikson R. L. Extracellular signals and reversible protein phosphorylation: what to Mek of it all. Cell. 1993 Jul 30;74(2):215–217. doi: 10.1016/0092-8674(93)90411-i. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993 Jun 17;363(6430):595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Elion E. A., Grisafi P. L., Fink G. R. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990 Feb 23;60(4):649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Errede B., Gartner A., Zhou Z., Nasmyth K., Ammerer G. MAP kinase-related FUS3 from S. cerevisiae is activated by STE7 in vitro. Nature. 1993 Mar 18;362(6417):261–264. doi: 10.1038/362261a0. [DOI] [PubMed] [Google Scholar]
- Errede B., Levin D. E. A conserved kinase cascade for MAP kinase activation in yeast. Curr Opin Cell Biol. 1993 Apr;5(2):254–260. doi: 10.1016/0955-0674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Gartner A., Nasmyth K., Ammerer G. Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes Dev. 1992 Jul;6(7):1280–1292. doi: 10.1101/gad.6.7.1280. [DOI] [PubMed] [Google Scholar]
- Gartner A., Nasmyth K., Ammerer G. Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes Dev. 1992 Jul;6(7):1280–1292. doi: 10.1101/gad.6.7.1280. [DOI] [PubMed] [Google Scholar]
- Gomez N., Traverse S., Cohen P. Identification of a MAP kinase kinase kinase in phaeochromocytoma (PC12) cells. FEBS Lett. 1992 Dec 21;314(3):461–465. doi: 10.1016/0014-5793(92)81527-s. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Irie K., Takase M., Lee K. S., Levin D. E., Araki H., Matsumoto K., Oshima Y. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol Cell Biol. 1993 May;13(5):3076–3083. doi: 10.1128/mcb.13.5.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Kosako H., Nishida E., Gotoh Y. cDNA cloning of MAP kinase kinase reveals kinase cascade pathways in yeasts to vertebrates. EMBO J. 1993 Feb;12(2):787–794. doi: 10.1002/j.1460-2075.1993.tb05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Force T. L., Rapp U. R., Bonventre J. V., Avruch J. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase-kinase. J Biol Chem. 1993 Jul 25;268(21):16009–16019. [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Irie K., Gotoh Y., Watanabe Y., Araki H., Nishida E., Matsumoto K., Levin D. E. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol Cell Biol. 1993 May;13(5):3067–3075. doi: 10.1128/mcb.13.5.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Levin D. E. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol. 1992 Jan;12(1):172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Gotoh Y., Nishida E. Phosphorylation of Xenopus mitogen-activated protein (MAP) kinase kinase by MAP kinase kinase kinase and MAP kinase. J Biol Chem. 1993 Feb 15;268(5):3277–3281. [PubMed] [Google Scholar]
- Nadin-Davis S. A., Nasim A. A gene which encodes a predicted protein kinase can restore some functions of the ras gene in fission yeast. EMBO J. 1988 Apr;7(4):985–993. doi: 10.1002/j.1460-2075.1988.tb02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi M., Pang L., Decker S. J., Saltiel A. R. Nerve growth factor stimulates the activities of the raf-1 and the mitogen-activated protein kinases via the trk protooncogene. J Biol Chem. 1992 Jul 25;267(21):14604–14610. [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993 Apr;13(4):2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes N., Connell L., Errede B. STE11 is a protein kinase required for cell-type-specific transcription and signal transduction in yeast. Genes Dev. 1990 Nov;4(11):1862–1874. doi: 10.1101/gad.4.11.1862. [DOI] [PubMed] [Google Scholar]
- Robbins D. J., Zhen E., Owaki H., Vanderbilt C. A., Ebert D., Geppert T. D., Cobb M. H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993 Mar 5;268(7):5097–5106. [PubMed] [Google Scholar]
- Seger R., Seger D., Lozeman F. J., Ahn N. G., Graves L. M., Campbell J. S., Ericsson L., Harrylock M., Jensen A. M., Krebs E. G. Human T-cell mitogen-activated protein kinase kinases are related to yeast signal transduction kinases. J Biol Chem. 1992 Dec 25;267(36):25628–25631. [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr Assay of yeast mating reaction. Methods Enzymol. 1991;194:77–93. doi: 10.1016/0076-6879(91)94008-z. [DOI] [PubMed] [Google Scholar]
- Stevenson B. J., Rhodes N., Errede B., Sprague G. F., Jr Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G protein. Genes Dev. 1992 Jul;6(7):1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Anderson N. G., Erickson A. K. Purification of mitogen-activated protein kinase from epidermal growth factor-treated 3T3-L1 fibroblasts. Methods Enzymol. 1991;200:342–351. doi: 10.1016/0076-6879(91)00151-l. [DOI] [PubMed] [Google Scholar]
- Teague M. A., Chaleff D. T., Errede B. Nucleotide sequence of the yeast regulatory gene STE7 predicts a protein homologous to protein kinases. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7371–7375. doi: 10.1073/pnas.83.19.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda L., Inoue Y. H., Yoo M. A., Mizuno M., Hata M., Lim Y. M., Adachi-Yamada T., Ryo H., Masamune Y., Nishida Y. A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell. 1993 Feb 12;72(3):407–414. doi: 10.1016/0092-8674(93)90117-9. [DOI] [PubMed] [Google Scholar]
- Warbrick E., Fantes P. A. The wis1 protein kinase is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991 Dec;10(13):4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Vincent L. A., Haystead C., Haystead T. A., Michel H., Hunt D. F., Lynch K. R., Sturgill T. W. Molecular structure of a protein-tyrosine/threonine kinase activating p42 mitogen-activated protein (MAP) kinase: MAP kinase kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):173–177. doi: 10.1073/pnas.90.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashar B. M., Kelley C., Yee K., Errede B., Zon L. I. Novel members of the mitogen-activated protein kinase activator family in Xenopus laevis. Mol Cell Biol. 1993 Sep;13(9):5738–5748. doi: 10.1128/mcb.13.9.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem. 1993 May 25;268(15):11435–11439. [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr protein phosphatase. J Biol Chem. 1993 Aug 5;268(22):16116–16119. [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J Biol Chem. 1993 Nov 15;268(32):23933–23939. [PubMed] [Google Scholar]