Abstract
The expression of homosynaptic long-term depression (LTD) is thought to mediate a crucial role in sustaining memory function. Our in vivo investigations of LTD expression at lateral (LPP) and medial perforant path (MPP) synapses in the dentate gyrus (DG) corroborate prior demonstrations that PP-DG LTD is difficult to induce in intact animals. In freely moving animals, LTD expression occurred inconsistently among LPP-DG and MPP-DG responses. Interestingly, following acute electrode implantation in anesthetized rats, low-frequency stimulation (LFS; 900 pulses, 1 Hz) promotes slow-onset LTP at both MPP-DG and LPP-DG synapses that utilize distinct induction mechanisms. Systemic administration of the N-methyl-d-aspartate (NMDA) receptor antagonist (+/−)-cyclopiperidine-6-piperiperenzine (CPP; 10 mg/kg) 90 min before LFS selectively blocked MPP-DG but not LPP-DG slow onset LTP, suggesting MPP-DG synapses express a NMDA receptor-dependent slow onset LTP whereas LPP-DG slow onset LTP induction is NMDA receptor independent. In experiments where paired-pulse LFS (900 paired pulses, 200-ms paired-pulse interval) was used to induce LTD, paired-pulse LFS of the LPP resulted in rapid onset LTP of DG responses, whereas paired-pulse LFS of the MPP induced slow onset LTP of DG responses. Although LTD observations were very rare following acute electrode implantation in anesthetized rats, LPP-DG LTD was demonstrated in some anesthetized rats with previously implanted electrodes. Together, our data indicate in vivo PP-DG LTD expression is an inconsistent phenomenon that is primarily observed in recovered animals, suggesting perturbation of the dentate through surgery-related tissue trauma influences both LTD incidence and LTP induction at PP-DG synapses in vivo.
Keywords: LTD, dentate gyrus, perforant path, slow onset LTP, in vivo
contrasting long-term potentiation (LTP), long-term depression (LTD) encompasses long lasting decreases in synaptic efficacy induced by low-frequency stimulation (LFS) (Barrionuevo et al. 1980; Dudek and Bear 1992). LTD is observed at many synapses, including the hippocampus (Dudek and Bear 1992; Mulkey and Malenka 1992; Bear and Abraham 1996), amygdala (Wang and Gean 1999; Lin et al. 2000; Heinbockel and Pape 2000), neocortex (Artola et al. 1990; Yoshimura et al. 1991; Kirkwood et al. 1993; Kirkwood and Bear 1994), and cerebellum (Ito 1986; Linden 1994). In addition to erasing memories (Stevens 1990; Siegelbaum and Kandel 1991; Tadaharu 1993; Malleret et al. 2010), preventing synaptic saturation in neural networks (Sejnowski 1977; Churchland and Sejnowski 1992; Rolls 1996), and increasing memory storage capacity (Willshaw and Dayan 1990; Churchland and Sejnowski 1992; Malenka 1994), mounting evidence suggest hippocampal LTD is critical for learning and encoding (Braunewell and Manahan-Vaughan 2001; Kemp and Manahan-Vaughn 2007, 2008), including object-place configuration memory (Kemp and Manahan-Vaughan 2004) and novelty acquisition (Manahan-Vaughan and Braunewell 1999; Lemon and Manahan-Vaughan 2006, 2012; Dong et al. 2012).
Although heterosynaptic LTD is routinely observed in the dentate gyrus (DG) in vivo (Abraham and Goddard 1983; Christie and Abraham 1992a; Doyère et al. 1997), homosynaptic perforant path-dentate gyrus (PP-DG) LTD has proved more elusive to elicit in the intact animal (Errington et al. 1995; Abraham 1996; Abraham et al. 1996; Doyère et al. 1996; Fung et al. 2011). LFS paradigms yielding consistent observations of LTD in CA1 are ineffective in producing DG LTD in vivo (Abraham et al. 1996; Doyère et al. 1996). For instance, some studies observe no DG homosynaptic LTD in vivo in response to LFS (Errington et al. 1995; Abraham et al. 1996), whereas Bramham and Srebro (1987) reported DG homosynaptic LTD in only 5 out of 16 intact animals following LFS. Furthermore, inducing PP-DG homosynaptic LTD in vivo has entailed a range of prerequisite manipulations, such as priming LTD by activating group 2 mGluRs (Manahan-Vaughan 1998) and stimulating DG afferents at an intensity evoking a less than half-maximal response (Klausnitzer et al. 2004; Pöschel and Manahan-Vaughan 2005, 2007).
DG afferents originate from lateral and medial entorhinal cortex, where inputs from these structures traverse the angular bundle via the lateral perforant path (LPP) and medial perforant path (MPP) before, respectively, forming synapses on distal and middle regions of DG granule cell dendrites (Hjorth-Simonsen 1972; Steward 1976; Witter et al. 1989; Witter and Amaral 2004; Wyss 1981). The LPP and MPP comprise physiologically distinct inputs (McNaughton 1980) that, respectively, relay nonspatial and spatial information to the hippocampal formation (Hargreaves et al. 2005; Hunsaker et al. 2007; Yoganarashima et al. 2011). The medial and lateral aspects of the PP were not explicitly isolated in some studies of in vivo DG homosynaptic LTD (Bramham and Srebro 1987; Errington et al. 1995; Abraham et al. 1996; Manahan-Vaughan 1998). This may have enabled mixed LPP and MPP activation. No investigations have sought to compare the relative propensity for LPP or MPP LFS to induce LTD in the DG in vivo during distinct behavioral states, including awake and pentobarbital or urethane-anesthetized states. Here, we compare in vivo homosynaptic LTD expression in LPP-DG and MPP-DG synapses following LFS in awake, freely moving animals or in intact animals under pentobarbital or urethane anesthesia. In freely moving animals, LTD expression occurred inconsistently among LPP-DG and MPP-DG responses. Interestingly, following acute electrode implantation in anesthetized rats, LFS (900 pulses, 1 Hz) promotes slow-onset LTP at both MPP-DG and LPP-DG synapses that utilize distinct induction mechanisms. In contrast, LPP-DG LTD was observed in four out of six anesthetized animals with previously implanted electrodes. Together, these data suggest in vivo PP-DG LTD is an inconsistent phenomenon that is primarily observed in animals with previously implanted electrodes, whereas LTP rather than LTD is observed in anesthetized animals following acute surgery. Thus these data suggest that surgery-related tissue trauma influences both in vivo LTD incidence and LTP induction at PP-DG synapses.
MATERIALS AND METHODS
Subjects.
The following experiments employed 106 adult male Sprague-Dawley rats (230–787 g, aged 3 to 9 mo old; Charles River Laboratories, Wilmington, MA) individually housed at the University of Texas at San Antonio animal facility under a 12-h light/dark cycle. Rats, aged 100 ± 6 postnatal days (means ± SE), were allowed ad libitum access to food and water before acute electrode implantation. Animals with chronic electrodes were older (165 ± 7 postnatal days old, means ± SE) at the time of experimentation as the stability of these electrodes requires no or minimal skull growth. These animals were fed a restricted diet to prevent excessive weight gain during postoperative recovery. All experimental procedures adhered to the National Institutes of Health Animal Care and Use Guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Texas at San Antonio.
Electrode implantation.
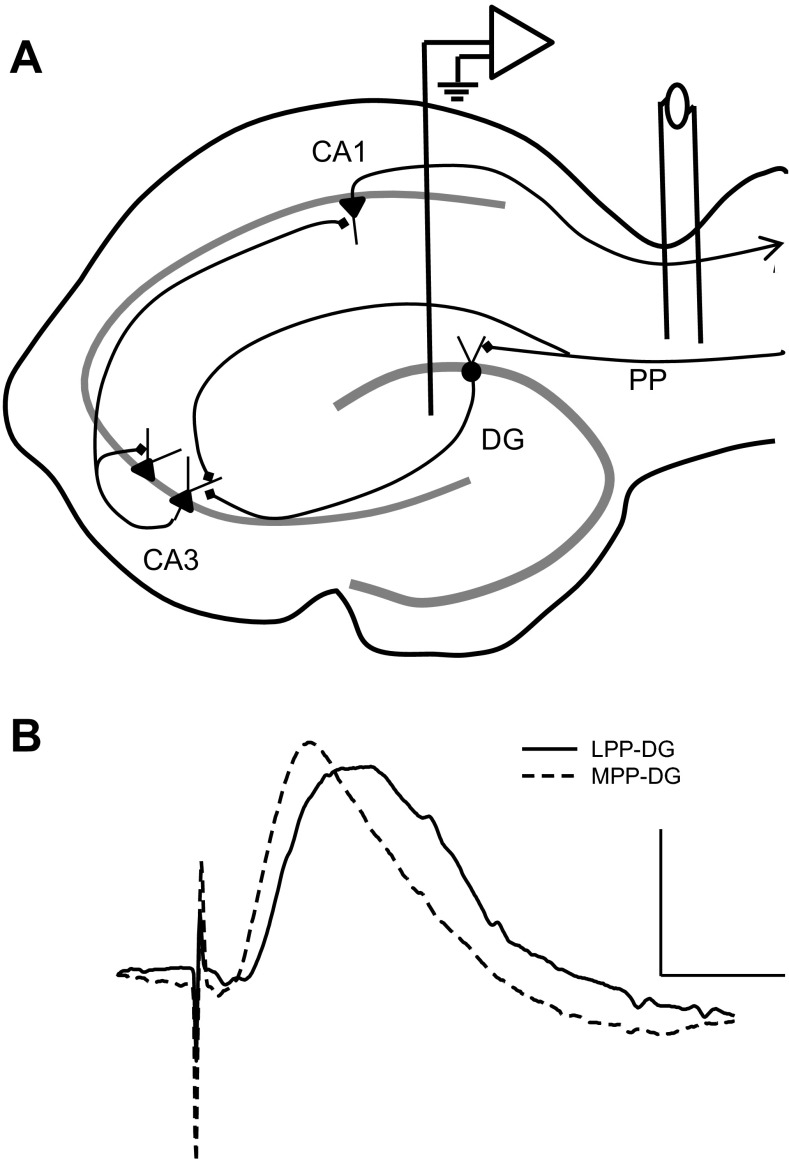
Rats were given surgical levels of anesthesia before electrode implantation under stereotaxic guidance. Specifically, acute experiments entailed anesthetizing rats (230–735 g) with either sodium pentobarbital (65 mg/kg Nembutal; Butler Schein) or urethane (1.5 g/kg; Sigma-Aldrich) administered intraperitoneal before implanting recording and stimulating electrodes, whereas chronic electrode implantation required sterile surgery in sodium pentobarbital-anesthetized rats. For surgeries involving sodium pentobarbital anesthesia, supplementary injections of pentobarbital sustained surgical levels of anesthesia. Body temperature was maintained at 37°C via a feedback controlled direct current heating pad. A Teflon-coated, stainless steel recording electrode (0.005 mm in diameter; A-M Systems) was lowered to the hilar region of the DG (A/P −3.5 mm, M/L +2.0 mm, D/V −3.0 mm; Paxinos and Watson 1994; Fig. 1A). Bipolar stainless steel stimulating electrodes were situated in either the MPP (A/P −7.9 mm, M/L +4.2 mm, D/V −2.5 mm; Paxinos and Watson 1994) or LPP (A/P −8.0 mm, M/L +5.0 mm, D/V −2.8 mm; Paxinos and Watson 1994) via placement in the dorsomedial or dorsolateral aspect of the angular bundle, respectively. DG responses were determined by utilizing both stereotaxic coordinates and audiolocalization of the CA1 pyramidal cell and DG granule cell layers. LPP-DG and MPP-DG responses exhibit different slope and peak latency characteristics (McNaughton and Barnes 1977; Abraham and McNaughton 1984; Bramham et al. 1991) that facilitate LPP-DG and MPP-DG response isolation. Specifically, LPP-DG responses display a decrease in slope and an increase in peak latency relative to MPP-DG responses (Fig. 1B; McNaughton and Barnes 1977; Abraham and McNaughton 1984; Bramham et al. 1991). Furthermore, MPP- and LPP-DG responses are distinguished on the marked paired-pulse facilitation observed in the LPP when stimulus intervals occur at 30–50 ms (McNaughton 1980; Andreasen and Hablitz 1994).
Fig. 1.
Isolating lateral perforant path-dentate gyrus (LPP-DG) and medial perforant path (MPP)-DG responses in vivo. A: schematic of electrode sites enabling the collection of PP-dentate gyrus responses. A recording electrode was implanted in the hilar region of the dentate gyrus and a bipolar stimulating electrode was positioned in either the lateral or medial aspect of the angular bundle to selectively stimulate LPP or MPP afferents, respectively. B: representative LPP-DG and MPP-DG responses indicate differences in peak latency, slope, and half height width of peak. In freely moving animals, LPP-DG responses (n = 4) exhibited an average peak latency of 6.1 ± 0.5 ms (means ± SE) measured from the trough of the stimulation artifact and a half height width of peak of 6.6 ± 0.9 ms, whereas MPP-DG responses (n = 6) demonstrated an average peak latency of 4.6 ± 0.5 ms and a half height width of peak of 4.6 ± 0.8 ms. Calibration: 0.5 mV, 5 ms.
In animals with permanent indwelling electrodes, the electrodes were attached with gold amphenol pins and stabilized in a head stage with stainless steel screws and dental acrylic. These animals received preoperative doses of atropine (0.1 mg/kg; Sigma-Aldrich) intraperitoneally to prevent respiratory congestion during surgery and an intramuscular injection of Aquacillin (100k units) or Baytril (enrofloxacin; 5 mg/kg) to prevent postoperative infection. Following permanent electrode implantation, rats were given Rimadyl (4 g, BID; BioServ), an analgesic, for 3 days postoperatively. Data collection ensued after a 2-wk recovery period.
Experimental design.
Behaving animal experiments were performed during the animal's light cycle and involved collecting responses evoked with current intensities that produce an approximation of the half-maximal peak slope, as determined by input/output assessments. In these experiments, LPP stimulation intensities ranged from 158 to 395 μA, whereas MPP stimulation intensities ranged from 171 to 495 μA. Rats were allowed to habituate to the recording chamber for at least 30 min before collecting PP evoked DG baseline field excitatory postsynaptic potential (EPSP) responses, thus limiting novelty-dependent facilitation of synaptic plasticity induction and maintenance (Davis et al. 2004; Lemon and Manahan-Vaughan 2006). Following a stable 20-min baseline, LFS (900 pulses, 1 Hz) was delivered to the PP and dentate responses were collected for an additional hour ensuing LFS.
Prior studies suggest LFS does not readily induce homosynaptic LTD in the DG (Errington et al. 1995; Abraham 1996; Abraham et al. 1996; Doyère et al. 1996) or in hippocampal area CA1 (Doyère et al. 1996, 1997) of intact animals. Furthermore, LTD induction by prolonged LFS is age delimited, occurring with less prevalence in adult animals (Dudek and Bear 1993; Kemp et al. 2000; Milner et al. 2004; Blaise and Bronzino 2003). Thus, following a stable 20-min baseline, acute experiments separately investigated the effects of three LTD induction paradigms on DG synaptic plasticity: 1) 900 pulses, 1-Hz LFS; 2) 900 paired-pulse LFS; and 3) 900 pulses, 1-Hz LFS delivered at below half-maximal stimulation intensities.
In experiments performed in anesthetized animals, half-maximal LPP-DG responses were evoked with a stimulation intensity range of 110–660 μA. MPP-DG responses were evoked with a half-maximal stimulation intensity range of 100–390 μA. Pentobarbital or urethane-anesthetized rats were given PP LFS (900 pulses, 1 Hz), and dentate responses were collected for an additional hour after LFS. In separate experiments performed under pentobarbital or urethane anesthesia, (+/−)-cyclopiperidine-6-piperiperenzine (CPP; 10 mg/kg; Tocris) was administered intraperitoneally 30 min before anesthesia and at least 90 min before LFS (900 pulses, 1 Hz). To assess whether afferent stimulus intensity influences PP-DG LTD expression, pentobarbital-anesthetized animals were given LPP or MPP LFS (900 pulses, 1 Hz) at current intensities yielding 25% of the maximal asymptotic DG field EPSP response. Thus low-intensity LPP LFS occurred at a current range of 140–435 μA, whereas low-intensity MPP LFS encompassed a current range of 22–245 μA. In these experiments, baseline drift was avoided by collecting a stable 30-min baseline of half-maximal DG field EPSP responses before collecting a 20-min baseline of responses evoked at low intensity. Other experiments involved stimulating LPP- or MPP-DG synapses with 900 paired pulses (1,800 stimuli, 200-ms paired-pulse interval) in pentobarbital-anesthetized rats. This paired-pulse LFS protocol is reported to induce LTD at CA3-CA1 synapses in adult rats in vitro (Kemp et al. 2000).
LTP occlusion experiments were performed in pentobarbital-anesthetized animals to compare the LTP expression mechanism mediating high-frequency stimulation-induced LTP and LFS-induced slow onset LTP. Following a stable 20-min baseline of MPP-DG responses evoked at half-maximal current intensity (110–520 μA), MPP fibers received three theta-burst stimulation (TBS) epochs occurring at 10-min intervals at current intensities producing 75% of the maximal asymptotic DG response (220–740 μA). Each TBS epoch consisted of five trains of ten 50-ms theta bursts delivered at 400 Hz every 200 ms and an intertrain interval of 20 s. Ensuing a 1-h MPP-DG response collection period after the last TBS epoch, LFS (900 pulses, 1 Hz) of the MPP preceded a subsequent hour long collection of post-LFS DG responses.
Dentate responses evoked from stimulating the PP at 0.066 Hz and were amplified, filtered at 0.3 Hz-10 kHz, digitized at 10 kHz, and stored for off-line analysis (DataWave Technologies, Loveland, CO). DG field EPSP slope measurements spanned a 1- to 2-ms period following response onset.
Data analysis.
All data were first normalized with respect to the average 10- to 15-min stable baseline field EPSP slope value. LFS-induced alterations in PP-DG synaptic efficacy were assessed via comparisons of the average field response slope measurements of the last 5 min of baseline and 56–60 min following LFS. Statistical analysis of LFS effects on PP-DG synaptic plasticity encompassed one-way repeated-measures ANOVA and post hoc Newman-Keuls test for pairwise comparisons with significance designated as P < 0.05. Correct electrode placement was verified by stereotaxic coordinates and audio localization of the CA1 pyramidal cell and DG granule cell layers. Furthermore, histologic analysis of DG electrode placement was carried out in a random sample of 73 out of 106 brains that were extracted after euthanizing rats with Beuthanasia (350 mg/kg sodium pentobarbital; Butler Schein) and subsequent decapitation.
RESULTS
LFS of the LPP or MPP inconsistently induces LTP in the DG of freely moving rats.
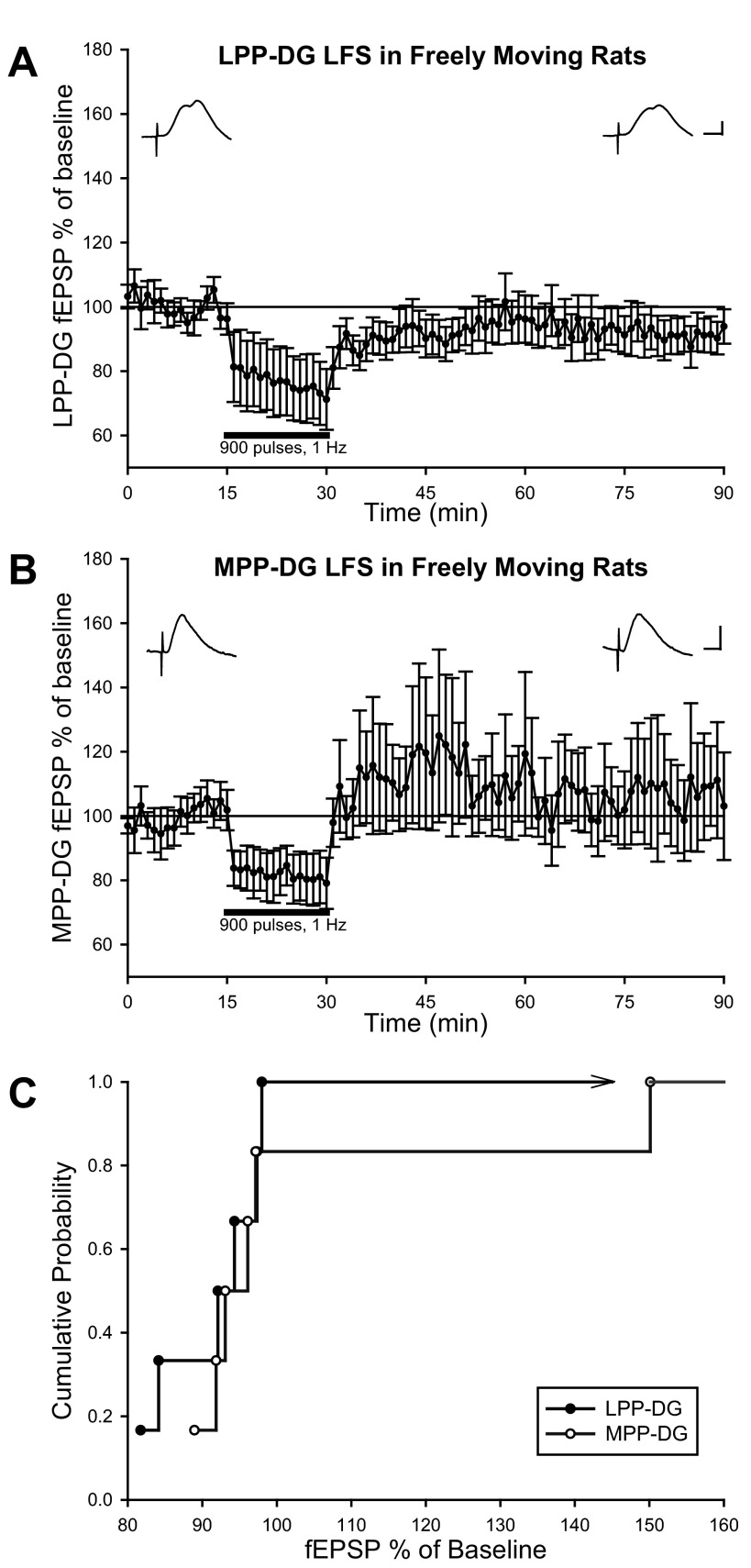
Table 1 summarizes the results of all PP-DG LFS data. To elucidate the relative propensity for LPP or MPP LFS to induce LTD in the DG of behaving rats, LFS-induced synaptic plasticity in the DG was compared in animals possessing a chronic stimulating electrode isolated in either the lateral or medial aspect of the angular bundle. Following LFS of the LPP (900 pulses, 1 Hz; n = 6), DG field EPSP slope measures exhibited a sustained decrease relative to baseline, lasting at least 1 h (91.3 ± 2.7%; Fig. 2A). A repeated-measures one-way ANOVA indicates LFS of the LPP produces a significant reduction in DG field EPSP [F(1,5) = 10.05, P < 0.05, n = 6; Fig. 2A] in awake, freely moving rats. Four out of six animals expressed homosynaptic LTD following LFS of the LPP, as assessed by significant differences among 5-min baseline and 56- to 60-min post-LFS slopes of individual experiments (P < 0.05, repeated-measures one-way ANOVA).
Table 1.
Perforant path-dentate gyrus low-frequency stimulation data summary
| Condition | LFS Paradigm | LPP/MPP | n | Normalized fEPSP 1-H Post-LFS |
|---|---|---|---|---|
| Awake, freely moving | 900 pulses, 1 Hz | LPP | 6 | 91.3 ± 2.7 |
| Awake, freely moving | 900 pulses, 1 Hz | MPP | 6 | 102.9 ± 10.4 |
| Pentobarbital | 900 pulses, 1 Hz | LPP | 5 | 109.4 ± 2.5 |
| CPP + pentobarbital | 900 pulses, 1 Hz | LPP | 6 | 118.5 ± 6.6 |
| Pentobarbital | 900 pulses, 1 Hz | MPP | 8 | 120.2 ± 7.1 |
| CPP + pentobarbital | 900 pulses, 1 Hz | MPP | 6 | 100.4 ± 4.7 |
| Urethane | 900 pulses, 1 Hz | LPP | 5 | 123.0 ± 4.2 |
| CPP + urethane | 900 pulses, 1 Hz | LPP | 7 | 110.9 ± 8.3 |
| Urethane | 900 pulses, 1 Hz | MPP | 5 | 132.2 ± 7.1 |
| CPP + urethane | 900 pulses, 1 Hz | MPP | 5 | 104.0 ± 9.6 |
| Pentobarbital | Low-intensity 900 pulses, 1 Hz | LPP | 6 | 111.5 ± 5.5 |
| Pentobarbital | Low-intensity 900 pulses, 1 Hz | MPP | 5 | 111.8 ± 9.0 |
| Pentobarbital | 900 paired pulses | LPP | 4 | 119.0 ± 6.7 |
| Pentobarbital | 900 paired pulses | MPP | 5 | 132.2 ± 10.6 |
| Pentobarbital (permanent electrodes) | 900 pulses, 1 Hz | LPP | 6 | 94.9 ± 9.1 |
| CPP + freely moving | 900 pulses, 1 Hz | LPP | 7 | 94.4 ± 8.4 |
| Vehicle + freely moving | 900 pulses, 1 Hz | LPP | 6 | 89.0 ± 12.1 |
Normalized field excitatory postsynaptic potentials (fEPSPs) reflect means ± SE. LFS, low-frequency stimulation; LPP, lateral perforant path; MPP, medial perforant path; CCP, (+/−)-cyclopiperidine-6-piperiperenzine.
Fig. 2.
Awake, freely moving rats display long-term depression (LTD) at LPP-DG synapses following low-frequency stimulation (LFS). A: LPP-DG field excitatory postsynaptic potential (fEPSP) dV/dt analysis expressed as a percent change of baseline (means ± SE). LPP LFS consisting of 900 pulses delivered at 1 Hz with stimulation intensities evoking a half-maximal LPP-DG response resulted in a sustained depression of DG fEPSPs in freely moving rats (n = 6). Calibration: 0.5 mV, 5 ms. B: normalized MPP-DG fEPSP dV/dt analysis (means ± SE) demonstrate 900 pulses, 1-Hz MPP LFS delivered at stimulation intensities evoking a half-maximal MPP-DG response generates no change in baseline DG fEPSPs in freely moving rats (n = 6). Calibration: 0.5 mV, 5 ms. C: empirical cumulative probability distributions of the alterations in dentate slopes following LPP or MPP LFS in freely moving rats. Each data point reflects the probability of individual dentate fEPSP ratios obtained from lateral or MPP LFS experiments. Dentate fEPSP ratios are derived from the average normalized slopes obtained 56–60 min post-LFS divided by the average normalized slopes collected 5 min before LFS.
Given the paucity of prior examinations of the receptors involved in homosynaptic LTD induction at LPP-DG synapses, separate experiments were conducted to evaluate the dependence of LTD on N-methyl-d-aspartate (NMDA) receptor activation. Animals were given either CPP (10 mg/kg), an NMDA receptor antagonist, or water vehicle at least 2 h before LFS of the LPP. Vehicle-treated animals (n = 6) demonstrated infrequent LPP-DG LTD expression following LFS, where only three out of six animals showed significant decreases in DG field EPSPs recorded 1 h after LPP LFS (P < 0.05, repeated-measures one-way ANOVA). In vehicle-treated animals, LPP-DG field EPSPs measured 89.0 ± 12.1% of baseline 1 h following LFS. Thus subsequent pharmacological analysis of LPP-DG LTD induction was not possible due a lack of significant depression in groupwise comparisons of LPP-DG field EPSPs recorded before and 1 h after LFS in vehicle-treated animals [F(1,5) = 1.01, P > 0.05, n = 6, repeated-measures one-way ANOVA]. These data suggest LPP-DG LTD induction was somewhat more reliable in animals that were not pretreated with a vehicle injection. In parallel experiments, CPP-treated animals (n = 7) exhibited DG field EPSPs measuring 94.4 ± 8.4% of baseline 1 h following LFS [F(1,6) = 0.65, P > 0.05, n = 7, repeated-measures one-way ANOVA]. Two out of seven CPP-treated animals displayed significant decreases in DG field EPSPs recorded 1 h after LPP LFS (P < 0.05, repeated-measures one-way ANOVA).
LFS of the MPP (900 pulses, 1 Hz) resulted in DG field EPSPs measuring 102.9 ± 10.4% of baseline [Fig. 2B; F(1,5) = 0.14, P > 0.05, n = 6, repeated-measures one-way ANOVA] in freely moving rats. Two out of six animals demonstrated significant reductions in MPP-DG field EPSPs recorded 1 h after MPP LFS (P < 0.05, repeated-measures one-way ANOVA). One out of six rats exhibited robust LTP following MPP LFS. Although more animals displayed significant reductions in PP-DG field EPSPs following LPP LFS relative to MPP LFS (P < 0.05, repeated-measures one-way ANOVA), Kolmogrov-Smirnov statistical analysis indicates no significant difference in empirical cumulative probability distributions of LFS-induced alterations in LPP-DG and MPP-DG slopes (P > 0.05; Fig. 2C). Thus physiological differences in LPP and MPP inputs (McNaughton 1980; Abraham and McNaughton 1984) do not impact the probability and magnitude of decreased PP-DG responses following LFS. Together, these data suggest LTD is induced inconsistently at both LPP-DG and MPP-DG synapses in freely moving animals.
LFS of the LPP or MPP promotes slow onset LTP in the DG of anesthetized rats.
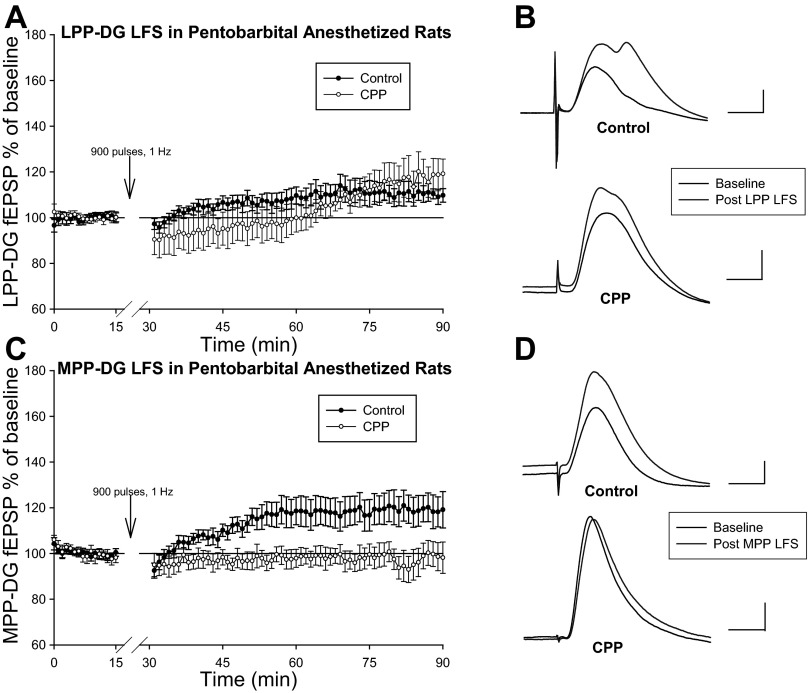
Evaluations of PP LFS in pentobarbital-anesthetized rats permitted additional analysis of dentate synaptic plasticity in intact animals. Although LTP is generally thought to occur with high-frequency stimulation (Bliss and Lomo 1973; Dudek and Bear 1992; Blaise and Bronzino 2003; Raymond 2007), previous studies demonstrate a slow-rising enhancement of field EPSPs following LFS (Li et al. 1998, 2001; Lanté et al. 2006a,b; Huang and Kandel 2007; Habib and Dringenberg 2009, 2010a). Similar to these studies, LFS (900 pulses, 1 Hz) of the LPP-induced slow onset LTP of dentate responses in sodium pentobarbital-anesthetized rats (n = 5; Li et al. 1998, 2001; Lanté et al. 2006a,b; Huang and Kandel 2007; Habib and Dringenberg 2009, 2010a). Following LFS, analysis of dentate field EPSPs indicates a significant change from baseline measuring 109.4 ± 2.5% [F(1,4) = 17.58, P < 0.05, n = 5, repeated-measures one-way ANOVA; Fig. 3, A and B]. Separate experiments similarly resulted in slow onset LTP [118.5 ± 6.6%, F(1,5) = 9.55, P < 0.05, n = 6, repeated-measures one-way ANOVA; Fig. 3, A and B] when the NMDA receptor antagonist CPP (10 mg/kg) was administered at least 90 min before LPP LFS in pentobarbital-anesthetized rats. Consistent with prior reports suggesting LPP-DG LTP occurs via a NMDA receptor-independent mechanism (Bramham et al. 1988, 1991), Newman-Keuls multiple pairwise comparisons characterize a significant increase in DG field EPSP following LFS of the LPP in CPP-treated pentobarbital-anesthetized rats. Furthermore, one-way ANOVA analysis revealed no significant difference between control and CPP-treated dentate field EPSP ratios derived from 56 to 60 min following LPP LFS and the last 5 min of baseline [F(1,9) = 1.72, P > 0.05] Thus, in contrast to the LTD observed in freely moving rats, LFS of the LPP promotes a slow onset LTP of DG field EPSPs in pentobarbital-anesthetized rats. Similar to LTP induced with high-frequency stimulation of the LPP, this slow onset LTP was NMDA receptor independent (Bramham et al. 1988, 1991).
Fig. 3.
LFS induces slow onset long-term potentiation (LTP) at LPP-DG and MPP-DG synapses in pentobarbital-anesthetized rats. A: comparison of normalized LPP-DG fEPSP dV/dt analysis (means ± SE) in control (n = 5) and (+/−)-cyclopiperidine-6-piperiperenzine (CPP)-treated (n = 6) pentobarbital-anesthetized rats. LPP LFS (900 pulses, 1 Hz) was delivered at stimulation intensities evoking a half-maximal LPP-DG response in both naïve rats and in animals given CPP at least 90 min before LFS. Both control and CPP-treated animals exhibit slow onset LTP following LPP LFS. B: representative LPP-DG fEPSPs recorded during baseline and 56–60 min after LPP LFS in control (top) and CPP-treated (bottom) pentobarbital-anesthetized rats. Calibration: 1 mV, 5 ms. C: assessment of normalized MPP-DG fEPSP dV/dt analysis (means ± SE) in control (n = 8) and CPP-treated (n = 6) pentobarbital-anesthetized rats. MPP LFS (900 pulses, 1 Hz) was delivered at stimulation intensities evoking a half-maximal MPP-DG response in both naïve rats and in animals given CPP at least 90 min before LFS. CPP blocked LFS-induced slow onset LTP at MPP-DG synapses. D: representative MPP-DG fEPSPs collected during baseline and 56–60 min after MPP LFS in control (top) and CPP-treated (bottom) pentobarbital-anesthetized rats. Calibration: 1 mV, 5 ms.
Four out of eight pentobarbital-anesthetized rats also expressed a MPP-DG slow onset LTP, defined as a slope increase of at least 20% above baseline sustaining for at least 1 h following LFS of the MPP (Fig. 3,C and D). Dentate field EPSPs measured 120.2 ± 7.1% of baseline [F(1,7) = 9.29, P < 0.05, n = 8, repeated-measures one-way ANOVA] after stimulating MPP fibers with 900 pulses at current intensities evoking a half-maximal response. Supporting previous studies demonstrating NMDA receptor-dependent LTP at MPP-DG synapses (Bramham et al. 1991), dentate field EPSPs exhibited no change relative to baseline when CPP administration preceded LFS of the MPP in pentobarbital-anesthetized rats [100.4 ± 4.7%, F(1,5) = 0.02, P > 0.05, n = 6, repeated-measures one-way ANOVA; Fig. 3, C and D]. Control and CPP-treated dentate field EPSP ratios derived from 56 to 60 min following MPP LFS and the last 5 min of baseline were significantly different [F(1,12)=5.27, P < 0.05, one-way ANOVA]. These data suggest NMDA receptors facilitate slow onset LTP induction following LFS of the MPP in pentobarbital-anesthetized rats.
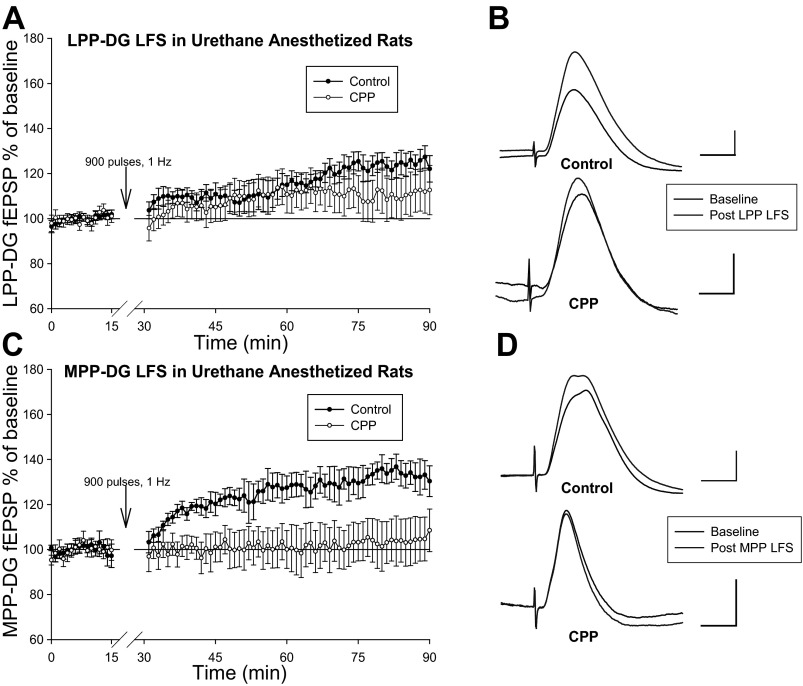
Given how urethane anesthesia poses relatively minimal influence on GABA inhibitory synaptic transmission (Maggi and Meli 1986; Sceniak and MacIver 2006), similar LFS experiments were performed in urethane-anesthetized rats to evaluate the possibility that pentobarbital-induced enhancement of GABA synaptic transmission (Schulz and MacDonald 1981; Study and Barker 1981; Twyman et al. 1989; Akaike et al. 1990; Eghbali et al. 2000, 2003; Birnir et al. 2000; Mathers et al. 2007) prevented DG LTD expression in pentobarbital-anesthetized rats. Urethane-anesthetized rats displayed slow onset LTP of DG field EPSPs lasting at least 1 h following LFS (900 pulses, 1 Hz) of the LPP [123 ± 4.2%, F(1,4) = 37.23, P < 0.05, n = 5, repeated-measures one-way ANOVA; Fig. 4, A and B]. Interestingly, LPP-DG slow onset LTP was not significant in additional studies involving CPP administration at least 90 min before LPP LFS in urethane-anesthetized rats [110.9 ± 8.3%, F(1,6) = 2.01, P > 0.05, n = 7, repeated-measures one-way ANOVA, Fig. 4, A and B]. In the presence of CPP, two out of seven urethane-anesthetized animals demonstrated LPP-DG slow onset LTP following LFS, suggesting NMDA receptor antagonists did not completely block LTP induction at these synapses. Nonetheless, the NMDA receptor sensitivity of slow-onset LTP at LPP-DG synapses in urethane-anesthetized rats is inconclusive given how control and CPP-treated LPP-DG field EPSP ratios demonstrated no significant difference in response to LPP LFS [F(1,10) = 1.54, P > 0.05, one-way ANOVA].
Fig. 4.
LFS induces slow onset LTP at LPP-DG and MPP-DG synapses in urethane-anesthetized rats. A: evaluation of normalized LPP-DG fEPSP dV/dt analysis (means ± SE) in control (n = 5) and CPP-treated (n = 7) urethane-anesthetized rats. LPP LFS (900 pulses, 1 Hz) was delivered at stimulation intensities evoking a half-maximal LPP-DG response in both naïve rats and in animals given CPP at least 90 min before LFS. Differences in post-LFS-to-baseline LPP-DG fEPSP ratios among control and CPP-treated rats were not statistically significant. B: representative LPP-DG fEPSPs collected during baseline and 56–60 min after LPP LFS in control (top) and CPP-treated (bottom) urethane-anesthetized rats. Calibration: 1 mV, 5 ms (top); 0.5 mV, 5 ms (bottom). C: comparison of normalized MPP-DG fEPSP dV/dt analysis (means ± SE) in control (n = 5) and CPP-treated (n = 5) urethane-anesthetized rats. MPP LFS (900 pulses, 1 Hz) was delivered at stimulation intensities evoking a half-maximal MPP-DG response in both naïve rats and in animals given CPP at least 90 min before LFS. CPP blocked LFS-induced slow onset LTP at MPP-DG synapses. D: representative MPP-DG fEPSPs recorded during baseline and 56–60 min after MPP LFS in control (top) and CPP-treated (bottom) urethane-anesthetized rats. Calibration: 1 mV, 5 ms.
Urethane-anesthetized animals showed a slow onset LTP of DG field EPSPs following LFS (900 pulses, 1 Hz) of the MPP. Specifically, normalized dentate field EPSPs measured 132.2 ± 7.1% of baseline 1 h after LFS of the MPP [F(1,4) = 26.37, P < 0.05, n = 5, repeated-measures one-way ANOVA; Fig. 4, C and D]. In the presence of CPP, LFS (900 pulses, 1 Hz) of the MPP produced no change in DG field EPSP in urethane-anesthetized rats [104 ± 9.6%, F(1,4) = 0.25, P > 0.05, n = 5, repeated-measures one-way ANOVA; Fig. 4, C and D]. One-way ANOVA analysis indicate a significant difference between control and CPP-treated DG response slope ratios derived from 56 to 60 min following MPP LFS and the last 5 min of baseline [F(1,8) = 7.27, P < 0.05]. Thus urethane-anesthetized rats also exhibit a NMDA receptor dependent slow onset LTP at MPP-DG synapses following LFS.
Taken together, in both pentobarbital- and urethane-anesthetized rats, LTD was not induced by LFS. Rather, a slow onset LTP of DG field EPSPs was observed in anesthetized rats following LFS of the MPP or LPP. Similar to the receptor sensitivities of MPP and LPP LTP induced by high-frequency stimulation (Bramham et al. 1991; Do et al. 2002), LFS-induced potentiation of MPP-DG synapses occurs via a NMDA receptor-dependent mechanism while LPP-DG slow onset LTP is induced in a NMDA receptor-independent manner in pentobarbital-anesthetized animals. Although NMDA receptor antagonists reduced the number of LPP-DG slow onset LTP observations in urethane-anesthetized animals, we were unable to determine the NMDA receptor sensitivity of LPP-DG slow onset LTP in these animals as LTP induction was not completely blocked by NMDA receptor antagonism. In lieu of how LPP-DG slow onset LTP was induced more reliably in CPP-treated pentobarbital, relative to CPP-treated urethane, anesthetized animals, anesthetic differences may influence the impact of NMDA receptor antagonists on LPP-DG slow onset LTP induction in response to LFS.
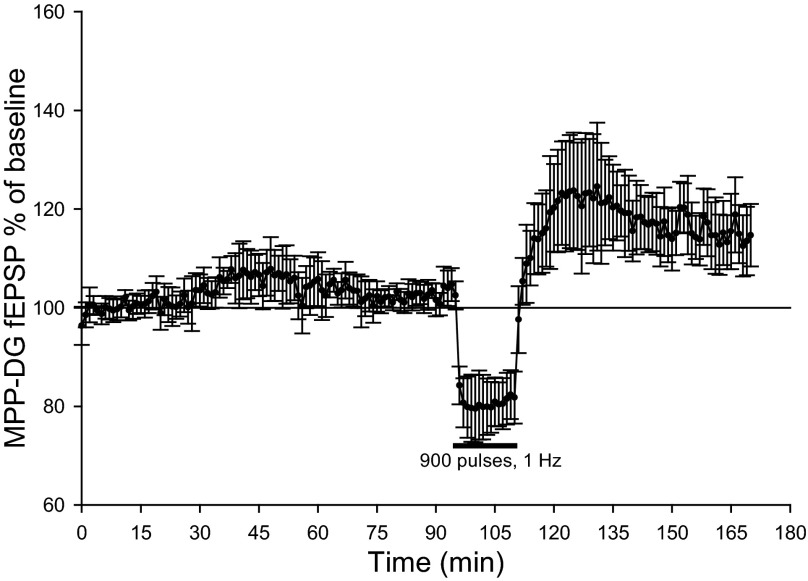
Prior examinations of PP-DG responses in vivo indicate baseline slope measures are slow to stabilize under pentobarbital or urethane anesthesia, where baselines slowly increased shortly after electrode implantation (Gilbert and Mack 1999). As the increases observed here similarly are slow to develop, we addressed whether these increases may be, rather than LTP, artifacts from electrode injury. In pentobarbital-anesthetized animals, we collected a stable 20-min baseline of MPP-DG responses before recording an additional 75 min of baseline responses, in lieu of the 15-min LFS and subsequent 1-h period of collection. Following this 75-min period, we delivered LFS (900 pulses, 1 Hz) and collected responses for an additional hour. As shown in Fig. 5, no increases in MPP-DG field EPSPs were observed over the 75-min period of extended baseline, where, before LFS, MPP-DG field EPSPs measured 101 ± 0.9% of initial baseline. In contrast, three out of three animals showed a significant increase in MPP-DG field EPSPs following LFS (P < 0.05, repeated-measures one-way ANOVA). One hour after LFS, MPP-DG field EPSPs measured 114 ± 6.6% of initial baseline. These data suggest PP-DG slow onset LTP requires activity-dependent effects of LFS in anesthetized animals.
Fig. 5.
Slow onset LTP requires LFS of PP afferents. Normalized MPP-DG fEPSPs indicate stable baseline recordings spanned over 90 min in pentobarbital-anesthetized rats (n = 3), suggesting our initial observations of slow onset LTP were not due to a slow rising baseline drift. Subsequent LFS (900 pulses, 1 Hz) induced a significant increase in MPP-DG fEPSPs recorded 1 h after LFS in 3 out of 3 animals (P < 0.05, repeated-measures one-way ANOVA).
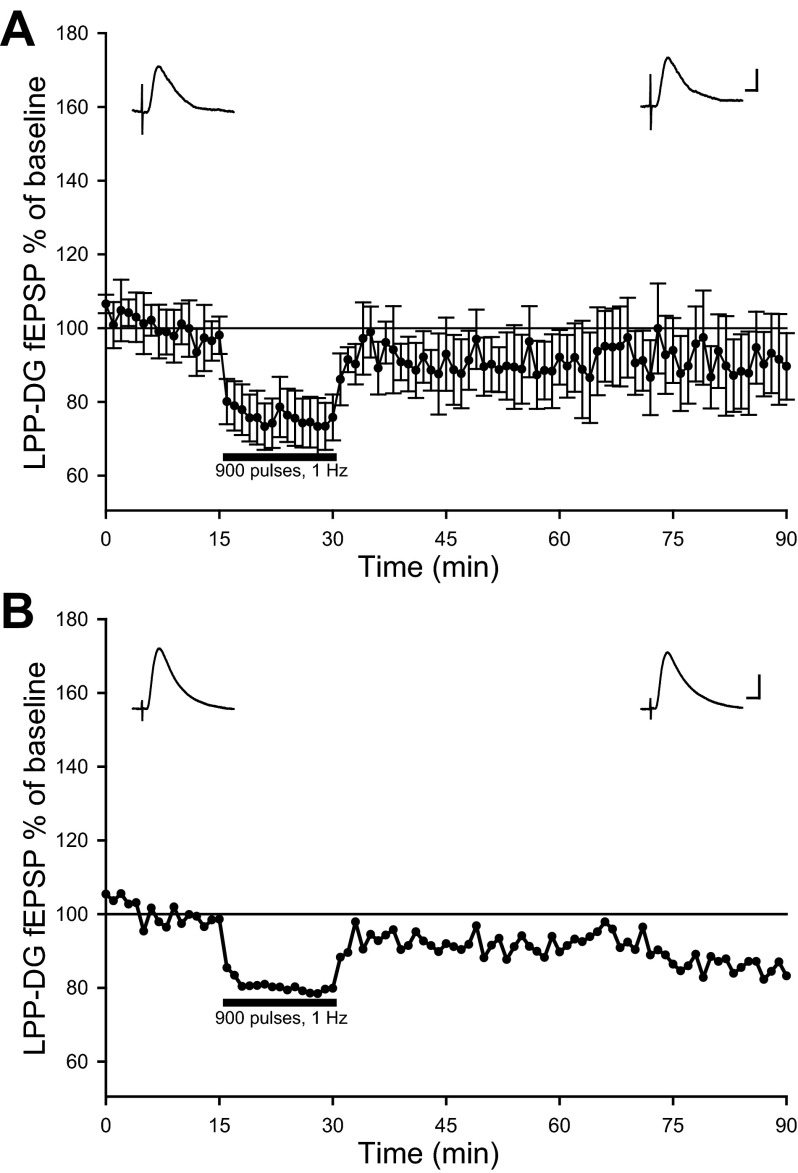
Previous studies indicate brief stress exposure suppressed homosynaptic LTD at LPP-DG synapses in ex vivo mouse hippocampal slices (Spyrka et al. 2011). Given the disparity of LPP-DG LTD observations in freely moving rats that did not occur in LFS experiments performed in anesthetized animals, we investigated the possibility that stress associated with acute surgery and stereotaxic restraint prevents LTD induction in anesthetized animals. To avoid the effects of acute electrode implantation required for in situ data collection in anesthetized animals (Jankowsky et al. 2000), rats implanted with electrodes 2 wk earlier were anesthetized with sodium pentobarbital (65 mg/kg ip) before subsequent LPP LFS (900 pulses, 1 Hz) in the absence of stereotaxic restraint. On average, LFS of the LPP produced no significant change in dentate field EPSPs [94.9 ± 9.1%, F(1,5) = 0.45, P > 0.05, n = 6, repeated-measures one-way ANOVA; Fig. 6A]. Interestingly, LFS of the LPP induced LTD in four out of six anesthetized rats with previously implanted electrodes (Fig. 6B), as assessed by significant differences among 5-min baseline and 56–60 min post-LFS slopes of individual experiments (P < 0.05, repeated-measures one-way ANOVA). The occurrence of LPP-DG LTD in four out of six experiments was observed in both freely moving and anesthetized rats with previously implanted electrodes. This contrasts with the very rare occurrence of LTD when LFS followed acute electrode implantation in anesthetized animals (4 out of 67 experiments). These data indicate LTD of LPP-DG synapses occurs with higher incidence in animals with previously implanted electrodes, suggesting surgery-related stress or tissue injury sustained during acute electrode implantation impairs LTD induction and concomitantly facilitates LTP induction in the DG.
Fig. 6.
LPP-DG LTD was observed in some anesthetized animals with previously implanted electrodes. A: averaged LPP-DG fEPSP dV/dt analysis in pentobarbital-anesthetized rats with previously implanted electrodes (n = 6) following 900 pulses, 1-Hz LPP LFS. B: data of an individual experiment demonstrating LTD at LPP-DG synapses in a pentobarbital-anesthetized rat with previously implanted electrodes. Similar LPP-DG LTD observations occurred in 4 out of 6 animals.
Low-intensity LFS of the LPP or MPP fails to elicit LTD in the DG of anesthetized rats.
Low-frequency afferent stimulation is thought to yield a small-to-moderate increase in intracellular calcium concentration that favors LTD induction (Lisman 1989; Mulkey and Malenka 1992; Artola and Singer 1993; Bear and Malenka 1994; Linden and Connor 1995; Christie et al. 1996; Coussens and Teyler 1996; Yang et al. 1999). Given how LTP induction occurs with large increases in intracellular calcium concentration (Lisman 1989; Artola and Singer 1993; Bear and Malenka 1994; Coussens and Teyler 1996; Yang et al. 1999), slow onset LTP following LFS may reflect a high afferent stimulation intensity that activated a threshold number of afferent fibers enabling sufficient calcium influx to induce LTP. We therefore used a low-intensity LFS paradigm (Klausnitzer et al. 2004; Pöschel and Manahan-Vaughan 2005, 2007) to investigate whether DG LTD induction requires subhalf-maximal afferent stimulus intensities (22–435 μA).
Following LFS (900 pulses, 1 Hz) of the LPP at current intensities evoking field EPSPs characterized as 25% of the maximal field EPSP, dentate field EPSPs measured 111.5 ± 5.5% of baseline (Fig. 7A) in sodium pentobarbital-anesthetized rats (n = 6). Interestingly, low-intensity LFS of the LPP produced a significant difference in dentate field EPSPs relative to baseline [F(1,5) = 7.48, P < 0.05, repeated-measures one-way ANOVA].
Fig. 7.
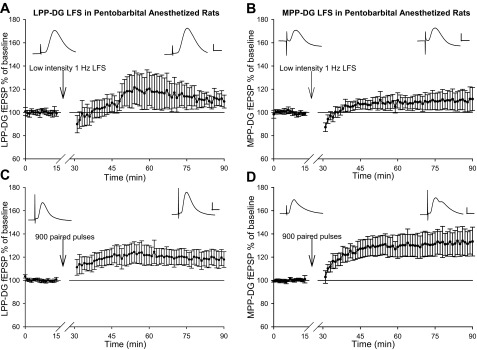
LFS at low current intensities and paired-pulse LFS fail to induce LTD at LPP-DG and MPP-DG synapses in pentobarbital-anesthetized rats. A: normalized LPP-DG fEPSP dV/dt analysis (means ± SE) demonstrate no LTD following 900 pulse, 1-Hz LPP LFS delivered at stimulation intensities evoking 25% of the maximal fEPSP response in pentobarbital-anesthetized rats (n = 6). Inset: responses calibration: 0.5 mV, 5 ms. B: normalized MPP-DG fEPSP dV/dt analysis (means ± SE) reveal no change in baseline MPP-DG fEPSPs following 900 pulse, 1-Hz MPP LFS delivered at stimulation intensities producing 25% of the maximal fEPSP response in pentobarbital-anesthetized rats (n = 5). Inset: calibration: 0.5 mV, 5 ms. C: normalized LPP-DG fEPSP dV/dt analysis (means ± SE) depicting the induction of a rapid onset LTP following LPP paired-pulse LFS (900 paired pulses, 200 ms interpulse interval) in pentobarbital-anesthetized rats (n = 4). Inset: calibration: 1 mV, 5 ms. D: normalized MPP-DG fEPSP dV/dt analysis (means ± SE) demonstrate slow onset LTP following MPP paired-pulse LFS (900 paired pulses, 200-ms interpulse interval) in pentobarbital-anesthetized rats (n = 5). Inset: response calibration: 1 mV, 5 ms.
Low-intensity LFS (900 pulses, 1 Hz) of the MPP produced no change in DG field EPSP relative to baseline in pentobarbital-anesthetized rats (111.8 ± 9.0%, P > 0.05, n = 5, Kruskal-Wallis one-way ANOVA on ranks; Fig. 7B). Taken together, these data suggest low-intensity LFS is insufficient to induce LPP- or MPP-DG LTD in anesthetized rats.
Paired-pulse LFS of the LPP or MPP does not induce LTP in the DG of anesthetized rats.
Previous studies demonstrate LTD induction by prolonged LFS (900 pulses, 1 Hz) occurs with less prevalence in adult animals (Dudek and Bear 1993; Kemp et al. 2000; Milner et al. 2004; Blaise and Bronzino 2003). Interestingly, paired-pulse LFS induces LTD at CA3-CA1 synapses in adult animals (Thiels et al. 1994; Kemp and Bashir 1997, 1999; Kemp et al. 2000). As we are using adult animals, we attempted to induce PP-DG LTD using a 900 paired-pulse LFS paradigm (1,800 stimuli, 200-ms paired-pulse interval) capable of inducing CA3-CA1 LTD in mature animals (Kemp et al. 2000). Stimulating the LPP with 900 paired pulses did not result in LTD of dentate field EPSPs in pentobarbital-anesthetized rats (n = 4). Instead, a rapid onset LTP of dentate field EPSPs ensued paired-pulse LFS of the LPP [Fig. 7C; F(1,3) = 10.54, P < 0.05, repeated-measures one-way ANOVA]. Potentiated LPP-DG responses measured 119 ± 6.7% of baseline field EPSPs and sustained without decay for at least 1 h.
Paired-pulse stimulation of the MPP pathway induced a slow onset LTP of DG field EPSPs in pentobarbital-anesthetized rats (Fig. 7D, n = 5). Dentate field EPSPs measured 132.2 ± 10.6% of baseline [F(1,4) = 10.93, P < 0.05, repeated-measures one-way ANOVA] following paired-pulse LFS of the MPP. The resulting slow onset LTP of MPP-DG synapses persisted at least 1 h without decrement. Together, these data suggest paired-pulse LFS of the LPP or MPP does not induce DG LTD in pentobarbital-anesthetized rats. Instead, PP paired-pulse LFS results in pathway specific variants of LTP in the DG.
Prior LTP saturation of PP-DG synapses occludes LFS-dependent slow onset potentiation in anesthetized rats.
LFS induces a slow onset LTP in anesthetized animals (Li et al. 1998, 2001; Lanté et al. 2006a, 2006b; Huang and Kandel 2007; Habib and Dringenberg 2009, 2010a). This potentiation is NMDA receptor dependent at MPP synapses and NMDA receptor independent at LPP synapses (Bramham et al. 1991). Because this parallels the NMDA dependence and independence of LTP at MPP and LPP synapses when using high-frequency stimulation (Bramham et al. 1991), this suggests the slow onset LTP induced by LFS and LTP induced by high-frequency stimulation employ similar expression mechanisms. If this is the case, high-frequency stimulation-induced LTP would be expected to occlude the subsequent expression of LFS-induced slow onset LTP (Habib and Dringenberg 2010a). In contrast, if these stimulation paradigms employ distinct LTP expression mechanisms, LTP induced by high-frequency stimulation will exhibit further potentiation following subsequent LFS.
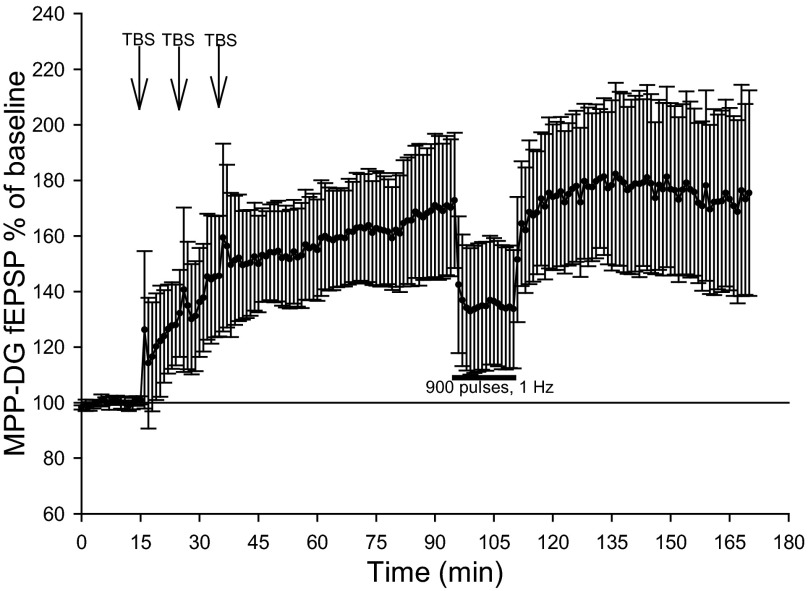
LTP occlusion experiments were performed in pentobarbital-anesthetized animals to compare the LTP expression mechanism mediating high-frequency stimulation-induced LTP and LFS-induced slow onset LTP. Theta burst stimulation of the MPP resulted in a robust LTP measuring 170.5 ± 34.7% of baseline dentate field EPSPs [F(1,4) = 9.99, P < 0.05, n = 5, repeated-measures one-way ANOVA; Fig. 8]. Subsequent LFS produced no further increase or decrease in post-TBS dentate field EPSPs (Fig. 8). Following LFS of the MPP, dentate field EPSPs measured 99.2 ± 7.5% of the average field EPSP occurring 5 min before LFS, indicating prior high-frequency stimulation-induced LTP occludes the subsequent occurrence of LFS-induced slow onset LTP in the DG. In addition, one-way ANOVA comparisons of the effects of LFS on dentate field EPSP ratios from pentobarbital-anesthetized rats given no prior TBS (n = 8) and those receiving LFS following previous theta burst stimulation (n = 5) approached significance [F(1,11)=4.41, P = 0.06, one-way ANOVA].
Fig. 8.
High-frequency stimulation-induced LTP occludes LFS-dependent slow onset LTP. Normalized MPP-DG fEPSP dV/dt analysis (means ± SE) depicts the expression of robust LTP following theta burst stimulation (5 trains of ten 50 ms, 400-Hz theta bursts with 20-s intertrain intervals repeated 3 times every 10 min) in pentobarbital-anesthetized rats (n = 5). Potentiated MPP-DG fEPSP exhibited no additional enhancement of fEPSP dV/dt following subsequent 900 pulse, 1-Hz MPP LFS.
These results demonstrate dentate LTP saturation mediated by theta burst stimulation of the MPP prevents LFS-induced slow onset potentiation in pentobarbital-anesthetized rats, suggesting LTP generated by TBS or LFS share common expression mechanisms, as is suggested for slow-onset LTP seen at other synapses (Habib and Dringenberg 2010a).
DISCUSSION
The present study is a systematic effort to evaluate the relative propensity for LPP or MPP LFS to induce homosynaptic LTD in the DG of both behaving rats and in sodium pentobarbital or urethane-anesthetized rats. Our data corroborate prior demonstrations that homosynaptic LTD is difficult to induce in the DG of intact animals (Errington et al. 1995; Abraham 1996; Abraham et al. 1996; Doyère et al. 1996; Fung et al. 2011). Our findings suggest dentate homosynaptic LTD is expressed inconsistently among both LPP-DG and MPP-DG synapses in freely moving animals. Interestingly, LTD was more likely to be observed in freely moving animals and anesthetized rats with previously implanted electrodes, whereas LTD was not observed when LFS followed acute electrode implantation in pentobarbital- or urethane-anesthetized rats. Rather than LTD, our acute experiments in anesthetized rats routinely resulted in a slow-rising LTP of MPP-DG and LPP-DG responses following LFS. Slow onset LTP induced by LFS in animals with previously implanted electrodes was rarely observed. Together, our findings suggest surgery-related tissue trauma produce perturbations that both impair LTD induction and facilitate the occurrence of LTP at PP-DG synapses in vivo.
LTD has traditionally been described as having either a homosynaptic or heterosynaptic origin. Homosynaptic LTD characterizes depression occurring at synapses receiving presynaptic LFS, whereas heterosynaptic LTD emanates at synapses that are not activated by high-frequency stimulation of inputs on the same postsynaptic neuron (Bear and Abraham 1996). Although heterosynaptic LTD is routinely observed in the DG in vivo (Abraham and Goddard 1983; Christie and Abraham 1992a; Doyère et al. 1997), homosynaptic PP-DG LTD has proved more elusive to elicit in the intact animal (Errington et al. 1995; Abraham 1996; Abraham et al. 1996; Doyère et al. 1996; Fung et al. 2011). Interestingly, Abraham et al. (2007) demonstrate heterosynaptic LTD requires presynaptic activity and thus may share similarities with homosynaptic LTD. Thus it remains unclear why homo- but not heterosynaptic LTD is difficult to obtain in vivo. Nevertheless, the difference in the prevalence of hetero- and homosynaptic LTD in the dentate may be explained as follows: as the induction of LTD requires moderate increases in intracellular calcium concentration (Lisman 1989; Mulkey and Malenka 1992; Artola and Singer 1993; Bear and Malenka 1994; Linden and Connor, 1995; Coussens and Teyler 1996; Yang et al. 1999), heterosynaptic LTD prevalence may relate to the propagating calcium waves produced by high-frequency stimulation (Jäger et al. 2002) at converging inputs. In this scenario, strongly stimulated synapses initiate a large intracellular increase in calcium concentration that may induce LTP (Lisman 1989; Artola and Singer 1993; Bear and Malenka 1994; Coussens and Teyler 1996; Yang et al. 1999). In addition, the calcium increase at strongly stimulated synapses may diffuse short distances within the dendrite (Jäger et al. 2002), allowing low-to-moderate intracellular calcium increases at adjacent synapses that may enable basal levels of presynaptic activity to induce “heterosynaptic” LTD (Abraham et al. 2007). Alternatively, feedforward inhibition of granule cell dendrites may limit the incidence of homosynaptic LTD by reducing PP postsynaptic depolarization. Given how presynaptic activity is required for LTD resulting from sustained low-frequency afferent stimulation or coincident LTP induction at adjacent afferents (Abraham et al. 2007), homosynaptic LTD may occur under different induction conditions. These variants of homosynaptic LTD may be dissociated in terms of whether LTD requires the co-occurrence of LTP at adjacent afferents. Nonetheless, the possibility that a given synapse expresses a single variant of homosynaptic LTD may explain the inconsistent observations of LTD following low-frequency afferent stimulation. Specifically, LTD expression that co-occurs with LTP induction at neighboring synapses may occlude subsequent LTD induced by low-frequency afferent stimulation.
In the present studies, LFS experiments ensuing acute electrode surgery in anesthetized animals failed to induce homosynaptic PP-DG LTD. Contrasting the dogma suggesting high-frequency stimulation elicits LTP whereas LFS induces LTD (Dudek and Bear 1992; Blaise and Bronzino 2003), our data indicate both pentobarbital- and urethane-anesthetized rats exhibit slow onset LTP (Li et al. 1998, 2001; Lanté et al. 2006a, 2006b; Huang and Kandel 2007; Habib and Dringenberg 2009, 2010a) of DG responses following 900 pulses, 1 Hz LFS of the LPP or MPP. We also demonstrate PP-DG slow onset LTP is not an artifact of a slow rising baseline previously observed in anesthetized animals (Gilbert and Mack 1999). Our findings of stable baselines lasting >90 min suggest DG slow onset LTP reflects an activity-dependent phenomenon that requires LFS. Although Lanté et al. (2006b) show 3- to 5-min short duration LFS is ineffective in eliciting DG slow onset LTP, previous reports demonstrate LFS-induced slow onset LTP occurring at external capsule-basolateral amygdala synapses after 900 pulses, 1 Hz LFS (Li et al. 1998, 2001; Huang and Kandel 2007), Schaffer collateral-CA1 synapses following 3- to 5-min short duration LFS (Lanté et al. 2006a, 2006b) and in response to alternating LFS of medial septal and CA3 commissural CA1 afferents (Habib and Dringenberg 2009, 2010a). Slow oscillations of similar ∼1 Hz frequency as these LFS paradigms have been reported in the hippocampus during non-REM sleep (Wolansky et al. 2006; Schall et al. 2008) and have been concomitantly implicated in memory consolidation (Bodizs et al. 2002; Marshall et al. 2006). Thus observations of LFS-induced synaptic potentiation in the hippocampus may suggest endogenous hippocampal slow oscillatory activity comprises a physiologically relevant framework enabling LTP and memory consolidation (Habib and Dringenberg 2010b). Similar to CA1 (Lanté et al. 2006b; Habib and Dringenberg 2010a,b), our data suggest the DG is capable of expressing several, distinct forms of slow onset LTP as evidenced by the presence of both NMDA receptor dependent and independent variants.
AMPA receptors mediate fast glutamatergic neurotransmission via GluA1–4 receptor subunits (Traynelis et al. 2010) that enable ligand gated sodium, potassium, and, depending on the receptor subunit composition, calcium conductance (Swanson et al. 1997). Phosphorylation of GluA1 receptor subunits has been implicated in enhancing AMPA receptor conductance (Derkach et al. 1999; Kristensen et al. 2011; Jenkins and Traynelis 2012) and regulating synaptic plasticity (Lee et al. 2000, 2003, 2010). Previous studies in knockout mice lacking GluA1 receptor subunits suggest paired theta burst stimulation at Schaffer collateral-CA1 synapses results in both a GluA1-dependent, rapid onset LTP and a GluA1-independent, slow onset LTP (Hoffman et al. 2002; Romberg et al. 2009). Specifically, stimulation paradigms resulting in rapid onset LTP may reflect the phosphorylation of GluA1 subunits and the subsequent increase in AMPA receptor conductance (Derkach et al. 1999; Kristensen et al. 2011; Jenkins and Traynelis 2012), augmenting the depolarization kinetics of rapid onset LTP. In contrast, LFS may trigger a GluA1-independent, slow onset LTP via slower mechanisms such as incorporating preexisting AMPA receptors in the postsynaptic density (Man et al. 2000; Malinow 2003; Hanley 2010). Thus observations of a rapid onset LTP may not preclude the parallel expression of an independent slow onset LTP (Habib and Dringenberg 2009). Nevertheless, the computational and functional differences between rapid and slow onset LTP remain undefined and warrants further study.
Similar to LTP occlusion studies in hippocampal area CA1 (Habib and Dringenberg 2010a), LFS-induced slow onset LTP at MPP-DG synapses is occluded by prior TBS-generated LTP in pentobarbital-anesthetized rats. LFS-induced LTP occlusion by prior HFS-induced LTP is thought to indicate both forms of LTP share a common expression mechanism (Habib and Dringenberg 2010a). However, it should be noted that LTP occlusion may also occur with DG activity-dependent modifications of subsequent plasticity induction efficacy and/or magnitude, a phenomenon known as metaplasticity (Abraham and Bear 1996; Abraham and Tate 1997; Christie and Abraham 1992b; Christie et al. 1995; Philpot et al. 1999; Derrick 2007). Specifically, prior synaptic activity that induces LTP may increase the threshold for a subsequent induction of LTP (Bienenstock et al. 1982; Abraham and Bear 1996; Abraham and Tate 1997; Philpot et al. 1999; Derrick 2007). Because such metaplastic effects would inhibit further potentiation, metaplasticity may limit the utility of LTP occlusion as a means of determining whether LTP generated by different induction protocols share a common expression mechanism.
Although LTD induction by prolonged LFS (900 pulses, 1 Hz) occur with less prevalence in adult animals (Dudek and Bear 1993; Kemp et al. 2000; Milner et al. 2004; Blaise and Bronzino 2003), paired-pulse LFS induces LTD at CA3-CA1 synapses in adult animals (Thiels et al. 1994; Kemp and Bashir 1997, 1999; Kemp et al. 2000). As we utilize adult animals, we attempted to induce PP-DG LTD in adult anesthetized rats with a paired-pulse LFS paradigm effective in eliciting LTD in adult CA1 hippocampal slices (Kemp et al. 2000). Consistent with prior examinations of short, low-frequency trains of paired PP stimuli on DG field EPSPs (Doyére et al. 1996), our data show PP paired-pulse LFS (900 paired stimuli, 200-ms paired-pulse interval) failed to induce homosynaptic LTD in the DG. Instead, paired-pulse LFS of the LPP resulted in rapid onset LTP of DG field potentials, whereas paired-pulse LFS of the MPP induced slow onset LTP of DG field potentials. Thus the subfields of the hippocampal formation demonstrate differences in synaptic plasticity induced by LFS (Abraham et al. 1996; Doyère et al. 1996; Lanté et al. 2006b; Fung et al. 2011).
Afferent stimulation intensity may serve to regulate the number of fibers that produce presynaptic action potentials and, by extension, the amount of postsynaptic depolarization and increase in postsynaptic calcium concentration, where the magnitude of postsynaptic calcium concentration regulates the induction of LTP or LTD at a given synapse (Lisman 1989; Mulkey and Malenka 1992; Bear and Malenka 1994; Coussens and Teyler 1996; Yang et al. 1999). For instance, low afferent stimulation intensity may activate fewer presynaptic axons resulting in moderate levels of postsynaptic depolarization and small increases in postsynaptic calcium concentration that result in LTD induction. Thus our observations of LTP following LFS in anesthetized animals may indicate that the postsynaptic depolarization was too great to induce LTD. We thus explored the hypothesis that subhalf-maximal afferent stimulus intensities optimize dentate LTD induction in anesthetized rats by producing decreased levels of postsynaptic depolarization and calcium influx (Lisman 1989; Mulkey and Malenka 1992; Bear and Malenka 1994; Christie et al. 1996; Coussens and Teyler 1996; Yang et al. 1999). Surprisingly, low-intensity LFS of the LPP or MPP failed to induce DG LTD in anesthetized rats, suggesting that even low-intensity LFS is insufficient to induce PP-DG LTD in anesthetized rats.
In contrast, homosynaptic LTD was observed, albeit inconsistently, in the DG of awake, freely moving rats following LFS of PP afferents. Specifically, four out of six naïve (noninjected) animals displayed significant reductions in DG responses following LFS of the LPP, and two out of six animals exhibited significant reductions in DG responses following LFS of the MPP. Interestingly, homosynaptic LTD induction was somewhat less reliable when a vehicle intraperitoneal injection preceded LFS in freely moving animals. The basis for the lower incidence of LTD expression in vehicle-treated animals vs. noninjected animals is unknown. Nonetheless, the possibility that stress of intraperitoneal injections may have suppressed LTD induction in vehicle-treated animals is unlikely given our observations of LTD in some recovered animals that were given intraperitoneal injections of pentobarbital anesthesia (see below).
Relative to the rare occurrence of LTD following acute electrode implantation in anesthetized rats, the greater incidence of homosynaptic PP-DG LTD in freely moving rats may result from either state-specific differences between anesthetized and behaving animals or the impaired LTD induction associated with surgery-related stress or tissue injury sustained during acute electrode implantation. These alternatives motivated our examinations of LFS-induced synaptic plasticity at LPP-DG synapses in anesthetized rats with previously implanted chronic recording and stimulating electrodes. Although significant LTD was not observed in our averaged data obtained from LFS of the LPP in anesthetized rats with previously implanted electrodes, four out of six anesthetized animals exhibited LTD following LFS. These data suggest state-specific differences between anesthetized and behaving animals are unlikely to explain the absence of LTD when LFS experiments immediately followed acute electrode implantation in anesthetized animals. Interestingly, slow onset LTP was rarely observed following LFS in anesthetized animals with previously implanted electrodes. Thus anesthesia effects are unlikely to facilitate the routine expression of LFS-induced slow onset LTP following acute electrode implantation in anesthetized rats. As our data show animals with previously implanted electrodes demonstrate a higher incidence of PP-DG LTD and rarely express slow onset LTP following LFS, it is possible that surgery-related stress or tissue injury sustained during acute electrode implantation may simultaneously impair LTD induction and facilitate LTP expression at PP-DG synapses. However, numerous studies show behavioral stress facilitates LTD induction in the dorsal hippocampus (Xu et al. 1997; Xiong et al. 2004; Yang et al. 2005; Artola et al. 2006; Chaouloff et al. 2007; Titterness and Christie 2008; Maggio and Segal 2009; Niehusmann et al. 2010). Thus stress-related effects of handling, administration of anesthetic, acute surgery, and stereotaxic restraint would be expected to facilitate LTD induction and cannot explain the impaired LTD induction observed in our acute LFS experiments. As stress is known to suppress LTP in the dorsal hippocampus (Foy et al. 1987; Shors et al. 1989, 1990; Diamond and Rose 1994; Shors and Dryver 1994; Garcia et al. 1997; Pavlides et al. 2002; Maroun and Richter-Levin 2003; Vouimba et al. 2004; Xiong et al. 2004; Howland and Wang 2008), our observations of slow onset LTP are unlikely due to stress-related effects of acute surgery and stereotaxic restraint. Alternatively, transient tissue trauma that ensues electrode implantation may encompass a more plausible reason for the diminished occurrence of LTD and the routine expression of slow onset LTP in our acute LFS experiments. Specifically, injury imposed by electrode penetration may result in a local immune response enabling glial release of prostaglandins and proinflammatory cytokines that subsequently induces hyperexcitability and disrupts neural plasticity (Jankowsky et al. 2000; Yirimya and Goshen 2011). In addition to suppressing LTD induction, inflammation-induced hyperexcitability may prime the induction of slow onset LTP following LFS of the PP as with prior studies showing enhanced cellular excitability primes LTP induction (Cohen et al. 1999). Jankowsky et al. (2000) demonstrate proinflammatory cytokine increases are not evident when animals are evaluated 3 wk after permanent electrode implantation. Thus recovery and the eventual decrease release of prostaglandins and proinflammatory cytokine production following tissue recovery in animals with previously implanted electrodes may allow dentate LTD induction.
In vitro techniques and in vivo electrode insertion both generate tissue trauma and proinflammatory cytokines (Jankowsky et al. 2000). Nonetheless, PP-DG homosynaptic LTD is routinely observed in vitro (Trommer et al. 1995; O'Mara et al. 1995; Wang et al. 1997), whereas PP-DG homosynaptic LTD is not readily induced in vivo (Errington et al. 1995; Abraham 1996; Abraham et al. 1996; Doyère et al. 1996; Fung et al. 2011). As LTD is primarily observed in young animals (Dudek and Bear 1993; Kemp et al. 2000; Milner et al. 2004; Blaise and Bronzino 2003), one possible explanation for this disparity may stem from the use of younger animals (aged ≤35 postnatal days old) in in vitro investigations (Trommer et al. 1995; O'Mara et al. 1995; Wang et al. 1997) compared with those of in vivo studies (Errington et al. 1995; Abraham et al. 1996; Doyère et al. 1996; Fung et al. 2011). Given prior demonstrations of age-dependent increases in proinflammatory cytokine levels (Takao et al. 1996; Murray and Lynch 1996; Ye and Johnson 1999), the younger tissue used to investigate DG LTD in vitro may have less susceptibility for trauma-related impairment of synaptic plasticity.
In summary, homosynaptic LTD expression in the DG is an inconsistent phenomenon observed primarily in freely moving and anesthetized animals with previously implanted electrodes. In contrast, following surgery and acute electrode implantation, anesthetized rats demonstrate LFS mediated slow-onset LTP at both MPP-DG and LPP-DG synapses that utilize distinct induction mechanisms. Together, these data demonstrate homosynaptic PP-DG LTD is observed more readily in recovered animals with previously implanted electrodes compared with animals with acute electrode surgery, suggesting surgery-related tissue trauma influence in vivo LTD incidence and LTP induction at PP-DG synapses. Future studies may evaluate whether homosynaptic LTD induction by LFS at LPP-DG and MPP-DG synapses is modulated by sleep-wake behavioral states, as with synaptic plasticity induced by high-frequency stimulation at PP-DG synapses (Bramham and Srebro 1989).
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke NS-066987 (to B. E. Derrick), National Institute of General Medical Sciences Grant MBRS-RISE-60655 (to J. Gonzalez), and Alfred P. Sloan Foundation (to J. Gonzalez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.G., I.S.M., D.M.V., and B.E.D. performed experiments; J.G. analyzed data; J.G. interpreted results of experiments; J.G. prepared Figs.; J.G. drafted manuscript; J.G., D.M.V., and B.E.D. edited and revised manuscript; J.G., I.S.M., D.M.V., and B.E.D. approved final version of manuscript; B.E.D. conception and design of research.
ACKNOWLEDGMENTS
We thank Julie Bland, DeNard Simmons, Soomin C. Song, Mahitha Raavi, and Jessica Briscoe for assistance in collecting some of the data. We also thank Dr. Helen Scharfman and Dr. Daijin Ko for advice and expertise.
REFERENCES
- Abraham WC, Goddard GV. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-term depression. Nature 305: 717–719, 1983 [DOI] [PubMed] [Google Scholar]
- Abraham WC, McNaughton N. Differences in synaptic transmission between medial and lateral components of the perforant path. Brain Res 303: 251–260, 1984 [DOI] [PubMed] [Google Scholar]
- Abraham WC. Induction of heterosynaptic and homosynaptic long-term depression in hippocampal subregions in vivo. J Physiol (Paris) 90: 305–306, 1996 [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996 [DOI] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Logan B. Low-frequency stimulation does not readily cause long-term depression or depotentiation in the dentate gyrus of awake rats. Brain Res 722: 217–221, 1996 [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol 52: 303–323, 1997 [DOI] [PubMed] [Google Scholar]
- Abraham WC, Logan B, Wolff A, Benuskova L. “Heterosynaptic” LTD in the dentate gyrus of anesthetized rat requires homosynaptic activity. J Neurophysiol 98: 1048–1051, 2007 [DOI] [PubMed] [Google Scholar]
- Akaike N, Tokutomi N, Ikemoto Y. Augmentation of GABA-induced current in frog sensory neurons by pentobarbital. Am J Physiol Cell Physiol 258: C452–C460, 1990 [DOI] [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Paired pulse facilitation in the dentate gyrus: a patch-clamp study in the rat hippocampus in vitro. J Neurophysiol 72: 326–336, 1994 [DOI] [PubMed] [Google Scholar]
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347: 369–372, 1990 [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16: 480–487, 1993 [DOI] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci 23: 261–272, 2006 [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci 27: 2385–2391, 1980 [DOI] [PubMed] [Google Scholar]
- Bear M, Abraham W. Long-term depression in hippocampus. Annu Rev Neurosci 19: 437–462, 1996 [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399, 1994 [DOI] [PubMed] [Google Scholar]
- Bienenstock E, Cooper L, Munro P. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 2: 32–48, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnir B, Eghbali M, Everitt AB, Gage PW. Bicuculline, pentobarbital and diazepam modulate spontaneous GABA(A) channels in rat hippocampal neurons. Br J Pharmacol 131: 695–704, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise JH, Bronzino JD. Effects of stimulus frequency and age on bidirectional synaptic plasticity in the dentate gyrus of freely moving rats. Exp Neurol 182: 497–506, 2003 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232: 331–356, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bǒdizs R, Békésy M, Szú́cs A, Barsi P, Halász P. Sleep-dependent hippocampal slow activity correlates with waking memory performance in humans. Neurobiol Learn Mem 78: 441–457, 2002 [DOI] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. Induction of long-term depression and potentiation by low- and high-frequency stimulation in the dentate area of the anesthetized rat: magnitude, time course and EEG. Brain Res 405: 100–107, 1987 [DOI] [PubMed] [Google Scholar]
- Bramham CR, Errington ML, Bliss TP. Naloxone blocks the induction of long-term potentiation in the lateral but not in the medial perforant pathway in the anesthetized rat. Brain Res 449: 352–356, 1988 [DOI] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Res 493: 74–86, 1989 [DOI] [PubMed] [Google Scholar]
- Bramham C, Milgram N, Srebro B. Activation of AP5-sensitive NMDA receptors is not required to induce LTP of synaptic transmission in the lateral perforant path. Eur J Neurosci 3: 1300–1308, 1991 [DOI] [PubMed] [Google Scholar]
- Braunewell K, Manahan-Vaughan D. Long-term depression: a cellular basis for learning? Rev Neurosci 12: 121–140, 2001 [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Hémar A, Manzoni O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. J Neurosci 27: 7130–7135, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie B, Abraham W. NMDA-dependent heterosynaptic long-term depression in the dentate gyrus of anaesthetized rats. Synapse 10: 1–6, 1992a [DOI] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. Priming of associative long-term depression in the dentate gyrus by theta frequency synaptic activity. Neuron 9: 79–84, 1992b [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Reduction of the threshold for long-term potentiation by prior theta-frequency synaptic activity. Hippocampus 5: 52–59, 1995 [DOI] [PubMed] [Google Scholar]
- Christie BR, Magee JC, Johnston D. The role of dendritic action potentials and Ca2+ influx in the induction of homosynaptic long-term depression in hippocampal CA1 pyramidal neurons. Learn Mem 3: 160–169, 1996 [DOI] [PubMed] [Google Scholar]
- Churchland PS, Sejnowski TJ. The Computational Brain. Cambridge, MA: MIT Press, 1992, p. 239–330 [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. J Neurophysiol 82: 3139–3148, 1999 [DOI] [PubMed] [Google Scholar]
- Coussens CM, Teyler TJ. Protein kinase and phosphatase activity regulate the form of synaptic plasticity expressed. Synapse 24: 97–103, 1996 [DOI] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. J Neurosci 24: 6497–6506, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96: 3269–3274, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick BE. Plastic processes in the dentate gyrus: a computational perspective. Prog Brain Res 163: 417–451, 2007 [DOI] [PubMed] [Google Scholar]
- Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann NY Acad Sci 746: 411–414, 1994 [DOI] [PubMed] [Google Scholar]
- Do VH, Martinez CO, Martinez JLJ, Derrick BE. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J Neurophysiol 87: 669–678, 2002 [DOI] [PubMed] [Google Scholar]
- Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J Neurosci 32: 11980–11990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyère V, Errington ML, Laroche S, Bliss TV. Low-frequency trains of paired stimuli induce long-term depression in area CA1 but not in dentate gyrus of the intact rat. Hippocampus 6: 52–57, 1996 [DOI] [PubMed] [Google Scholar]
- Doyère V, Srebro B, Laroche S. Heterosynaptic LTD in the medial perforant path of the dentate gyrus in awake rats. J Neurophysiol 77: 571–578, 1997 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA 89: 4363–4367, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci 13: 2910–2918, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali M, Gage PW, Birnir B. Pentobarbital modulates gamma-aminobutyric acid-activated single-channel conductance in rat cultured hippocampal neurons. Mol Pharmacol 58: 463–469, 2000 [PubMed] [Google Scholar]
- Eghbali M, Gage PW, Birnir B. Conductance of GABAA channels activated by pentobarbitone in hippocampal neurons from newborn rats. J Physiol 552: 13–22, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington ML, Bliss TV, Richter-Levin G, Yenk K, Doyere V, Laroche S. Stimulation at 1–5 Hz does not produce long-term depression or depotentiation in the hippocampus of the adult rat in vivo. J Neurophysiol 74: 1793–1799, 1995 [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol 48: 138–149, 1987 [DOI] [PubMed] [Google Scholar]
- Fung TK, Peloquin P, Wu K, Leung LS. Differential long-term depression in CA3 but not in dentate gyrus following low-frequency stimulation of the medial perforant path. Synapse 65: 677–686, 2011 [DOI] [PubMed] [Google Scholar]
- Garcia R, Musleh W, Tocco G, Thompson RF, Baudry M. Time-dependent blockade of STP and LTP in hippocampal slices following acute stress in mice. Neurosci Lett 233: 41–44, 1997 [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM. Field potential recordings in dentate gyrus of anesthetized rats: stability of baseline. Hippocampus 9: 277–287, 1999 [DOI] [PubMed] [Google Scholar]
- Habib D, Dringenberg HC. Alternating low frequency stimulation of medial septal and commissural fibers induces NMDA-dependent, long-lasting potentiation of hippocampal synapses in urethane-anesthetized rats. Hippocampus 19: 299–307, 2009 [DOI] [PubMed] [Google Scholar]
- Habib D, Dringenberg HC. Surprising similarity between mechanisms mediating low (1 Hz)-and high (100 Hz)-induced long-lasting synaptic potentiation in CA1 of the intact hippocampus. Neuroscience 170: 489–496, 2010a [DOI] [PubMed] [Google Scholar]
- Habib D, Dringenberg HC. Low-frequency-induced synaptic potentiation: a paradigm shift in the field of memory-related plasticity mechanisms? Hippocampus 20: 29–35, 2010b [DOI] [PubMed] [Google Scholar]
- Hanley JG. Endosomal sorting of AMPA receptors in hippocampal neurons. Biochem Soc Trans 38: 460–465, 2010 [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308: 1792–1794, 2005 [DOI] [PubMed] [Google Scholar]
- Heinbockel T, Pape HC. Input-specific long-term depression in the lateral amygdala evoked by theta frequency stimulation. J Neurosci 20: RC68, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth-Simonsen A. Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentate. J Comp Neurol 146: 219–232, 1972 [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Sprengel R, Sakmann B. Molecular dissection of hippocampal theta-burst pairing potentiation. Proc Natl Acad Sci USA 99: 7740–7745, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: Stress effects in the hippocampus. Prog Brain Res 169: 145–158, 2008 [DOI] [PubMed] [Google Scholar]
- Huang Y, Kandel ER. Low-frequency stimulation induces a pathway-specific late phase of LTP in the amygdala that is mediated by PKA and dependent on protein synthesis. Learn Mem 14: 497–503, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner R. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci 121: 742–750, 2007 [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression as a memory process in the cerebellum. Neurosci Res 3: 531–539, 1989 [DOI] [PubMed] [Google Scholar]
- Jäger T, Behnich T, Reymann KG. High-frequency stimulation-induced dendritic calcium waves in rat hippocampal neurons. Neurosci Lett 335: 103–106, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learn Mem 7: 400–412, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Traynelis SF. PKC phosphorylates GluA1-Ser831 to enhance AMPA receptor conductance. Channels 6: 60–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. NMDA receptor-dependent and -independent long-term depression in the CA1 region of the adult rat hippocampus in vitro. Neuropharmacology 36: 397–399, 1997 [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology 38: 495–504, 1999 [DOI] [PubMed] [Google Scholar]
- Kemp N, McQueen J, Faulkes S, Bashir ZI. Different forms of LTD in the CA1 region of the hippocampus: role of age and stimulus protocol. Eur J Neurosci 12: 360–366, 2000 [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA 101: 8192–8197, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci 30: 111–118, 2007 [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex 18: 968–977, 2008 [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Alzenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science 260: 1518–1521, 1993 [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci 14: 3404–3412, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausnitzer J, Kulla A, Manahan-Vaughan D. Role of the group III metabotropic glutamate receptor in LTP, depotentiation and LTD in dentate gyrus of freely moving rats. Neuropharmacology 46: 160–170, 2004 [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci 14: 727–735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanté F, Cavalier M, Cohen-Solal C, Guiramand J, Vignes M. Developmental switch from LTD to LTP in low frequency-induced plasticity. Hippocampus 16: 981–989, 2006a [DOI] [PubMed] [Google Scholar]
- Lanté F, de Jesus Ferreira MC, Guiramand J, Recasens M, Vigens M. Low-frequency stimulation induces a new form of LTP, metabotropic glutamate (mGlu5) receptor- and PKA-dependent, in the CA1 area of the rat hippocampus. Hippocampus 16: 345–360, 2006b [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Hugnair RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405: 955–959, 2000 [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643, 2003 [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol 103: 479–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci 26: 7723–7729, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors contribute to de novo LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex 22: 2131–2138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Weiss SR, Chuang DM, Post RM, Rogawski MA. Bidirectional synaptic plasticity in the rat basolateral amygdala: characterization of an activity-dependent switch sensitive to the presynaptic metabotropic glutamate receptor antagonist 2S-alpha-ethylglutamic acid. J Neurosci 18: 1662–1670, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen A, Xing G, Wei ML, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci 4: 612–620, 2001 [DOI] [PubMed] [Google Scholar]
- Lin HC, Wang SJ, Luo MZ, Gean PW. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J Neurosci 20: 9017–9024, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ. Induction of cerebellar long-term depression does not require pre-synaptic alteration. Learn Mem 1: 121–128, 1994 [PubMed] [Google Scholar]
- Linden D, Connor J. Long-term synaptic depression. Annu Rev Neurosci 18: 319–357, 1995 [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA 86: 9574–9578, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia 42: 109–114, 1986 [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. J Neurosci 29: 8633–8638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell 76: 535–538, 1994 [DOI] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358: 707–714, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, Chen IZ, Kandel ER, Shumyatsky GP. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci 30: 3813–3825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Ju W, Ahmadian G, Wang YT. Intracellular trafficking of AMPA receptors in synaptic plasticity. Cell Mol Life Sci 57: 1526–1534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Priming of group 2 metabotropic glutamate receptors facilitates induction of long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology 37: 1459–1464, 1998 [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell K. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA 96: 8739–8744, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 23: 4406–4409, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613, 2006 [DOI] [PubMed] [Google Scholar]
- Mathers DA, Wan X, Puil E. Barbiturate activation and modulation of GABA(A) receptors in neocortex. Neuropharmacology 52: 1160–1168, 2007 [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol 175: 439–454, 1977 [DOI] [PubMed] [Google Scholar]
- McNaughton B. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res 199: 1–19, 1980 [DOI] [PubMed] [Google Scholar]
- Milner AJ, Cummings DM, Spencer JP, Murphy KP. Bi-directional plasticity and age-dependent long-term depression at mouse CA3-CA1 synapses. Neurosci Lett 367: 1–5, 2004 [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9: 967–795, 1992 [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci 18: 2974–2981, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehusmann P, Seifert G, Clark K, Atas HC, Herpfer I, Fiebich B, Bischofberger J, Normann C. Coincidence detection and stress modulation of spike time-dependent long-term depression in the hippocampus. J Neurosci 30: 6225–6230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mara SM, Rowan MJ, Anwyl R. Metabotropic glutamate receptor-induced homosynaptic long-term depression and depotentiation in the dentate gyrus of the rat hippocampus in vitro. Neuropharmacology 34: 983–989, 1995 [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivón LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus 12: 245–257, 2002 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic Atlas of the Rat Brain (2nd ed). New York: Academic, 1994 [Google Scholar]
- Philpot BD, Bear MF, Abraham WC. Metaplasticity: the plasticity of synaptic plasticity. In: Beyond Neurotransmission: Neuromodulation and its Importance for Information Processing, edited by Katz PS. Oxford Univ Press, 1999, p. 160–197 [Google Scholar]
- Pöschel B, Manahan-Vaughan D. Group II mGluR-induced long term depression in the dentate gyrus in vivo is NMDA receptor-independent and does not require protein synthesis. Neuropharmacology 49: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- Pöschel B., Manahan-Vaughan D. Persistent (>24 h) long-term depression in the dentate gyrus of freely moving rats is not dependent on activation of NMDA receptors, L-type voltage-gated calcium channels or protein synthesis. Neuropharmacology 52: 46–54, 2007 [DOI] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci 30: 167–175, 2007 [DOI] [PubMed] [Google Scholar]
- Rolls ET. Roles of LTP and LTD in neuronal network operations in the brain. In: Cortical Plasticity, edited by Fazeli MS, Collingridge GL. Oxford, UK: Bios, 1996, p. 223–250 [Google Scholar]
- Romberg C, Raffel J, Martin L, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM, Paulsen O. Induction and expression of GluA1 (GluR-A)-independent LTP in the hippocampus. Eur J Neurosci 29: 1141–1152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceniak MP, MacIver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol 95: 3865–3874, 2006 [DOI] [PubMed] [Google Scholar]
- Schall KP, Kerber J, Dickson CT. Rhythmic constraints on hippocampal processing: state and phase-related fluctuations of synaptic excitability during theta and the slow oscillation. J Neurophysiol 99: 888–899, 2008 [DOI] [PubMed] [Google Scholar]
- Schulz DW, MacDonald RL. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res 209: 177–188, 1981 [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ. Statistical constraints on synaptic plasticity. J Theor Biol 69: 385–389, 1977 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable vs. escapable shock modulates long-term potentiation in the rat hippocampus. Science 244: 224–226, 1989 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Foy MR, Levine S, Thompson RF. Unpredictable and uncontrollable stress impairs neuronal plasticity in the rat hippocampus. Brain Res Bull 24: 663–667, 1990 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Dryver E. Effect of stress and long-term potentiation (LTP) on subsequent LTP and the theta burst response in the dentate gyrus. Brain Res 666: 232–238, 1994 [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 1: 113–120, 1991 [DOI] [PubMed] [Google Scholar]
- Spyrka J, Danielewicz J, Hess G. Brief neck restraint stress enhances long-term potentiation and suppresses long-term depression in the dentate gyrus of the mouse. Brain Res Bull 85: 363–367, 2011 [DOI] [PubMed] [Google Scholar]
- Stevens CF. A depression long awaited. Nature 347: 16, 1990 [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167: 285–314, 1976 [DOI] [PubMed] [Google Scholar]
- Study RE, Barker JL. Diazepam and (–)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci USA 78: 7180–7184, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17: 58–69, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadaharu T. Long-term depression in cerebral cortex: a possible substrate of “forgetting” that should not be forgotten. Neurosci Res 16: 263–270, 1993 [DOI] [PubMed] [Google Scholar]
- Takao T, Nagano I, Tojo C, Takemura T, Makino S, Hashimoto K, De Souza EB. Age-related reciprocal modulation of interleukin-1beta and interleukin-1 receptors in the mouse brain-endocrine-immune axis. Neuroimmunomodulation 3: 205–212, 1996 [DOI] [PubMed] [Google Scholar]
- Thiels E, Barrionuevo G, Berger TW. Excitatory stimulation during postsynaptic inhibition induces long-term depression in hippocampus in vivo. J Neurophysiol 72: 3009–3016, 1994 [DOI] [PubMed] [Google Scholar]
- Titterness AK, Christie BR. Long-term depression in vivo: effects of sex, stress, diet, and prenatal ethanol exposure. Hippocampus 18: 481–91, 2008 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Kennelly JJ, Colley PA, Overstreet LS, Slater NT, Pasternak JF. AP5 blocks LTP in developing rat dentate gyrus and unmasks LTD. Exp Neurol 131: 83–92, 1995 [DOI] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, MacDonald RL. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci Lett 96: 89–95, 1989 [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Diamond D, Richter-Levin G. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur J Neurosci 19: 1887–1894, 2004 [DOI] [PubMed] [Google Scholar]
- Wang SJ, Gean PW. Long-term depression of excitatory synaptic transmission in the rat amygdala. J Neurosci 19: 10656–10663, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rowan MJ, Anwyl R. Induction of LTD in the dentate gyrus in vitro is NMDA receptor independent, but dependent on Ca2+ influx via low-voltage-activated Ca2+ channels and release of Ca2+ from intracellular stores. J Neurophysiol 77: 812–825, 1997 [DOI] [PubMed] [Google Scholar]
- Willshaw D, Dayan P. Optimal plasticity from matrix memories: what goes up must come down. Neural Comp 2: 85–93, 1990 [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9: 216–228, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: The Rat Nervous System (3rd ed), edited by Paxinos G. Amsterdam, The Netherlands: Elsevier Academic, 2004, p. 635–704 [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci 26: 6213–6229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol 199: 495–512, 1981 [DOI] [PubMed] [Google Scholar]
- Xiong W, Wei H, Xiang X, Cao J, Dong Z, Wang Y, Xu T, Xu L. The effect of acute stress on LTP and LTD induction in the hippocampal CA1 region of anesthetized rats at three different ages. Brain Res 1005: 497–502, 2004 [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 387: 497–500, 1997 [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol 81: 781–787, 1999 [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci 25: 4288–4292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol 93: 139–148, 1999 [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181–213, 2011 [DOI] [PubMed] [Google Scholar]
- Yoganarasimha D, Rao G, Knierim JJ. Lateral entorhinal neurons are not spatially selective in cue-rich environments. Hippocampus 21: 1363–1374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Tsumoto T, Nishigori A. Input-specific induction of long-term depression in Ca(2+)-chelated visual cortex neurons. Neuroreport 2: 393–396, 1991 [DOI] [PubMed] [Google Scholar]