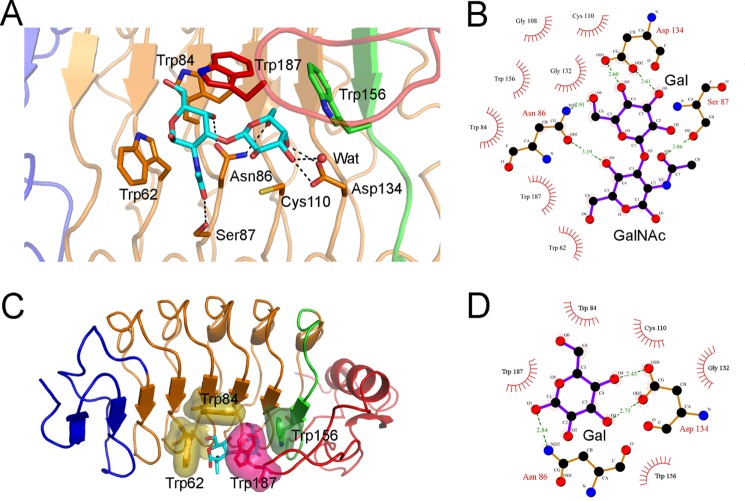
FIGURE 4.
The VLRB.aGPA.23-TFα binding interface. A, shown is a close-up view of VLRB.aGPA.23 residues involved in recognizing the TFα disaccharide. Colors (including residue labels) are as in Fig. 3. The side chains of contacting residues are drawn in stick representation with nitrogens in blue, oxygens in red, and sulfur in yellow. Hydrogen bonds are indicated by dotted lines. A water molecule bound to galactose O3′ is represented as a red sphere. B, shown is a schematic representation of interactions between VLRB.aGPA.23 and TFα. Residues making van der Waals contacts with TFα are indicated by arcs with spokes radiating toward the ligand moieties they contact. C, shown is tight packing of hydrophobic residues Trp-62, Trp-84, Trp-156, and Trp-187 around TFα, with their van der Waals radii drawn as surfaces. D, shown are schematic representations of interactions between VLRB.aGPA.23 and BG-H.