Abstract
Animal flight at altitude involves substantial aerodynamic and physiological challenges. Hovering at high elevations is particularly demanding from the dual perspectives of lift and power output; nevertheless, some volant insects reside and fly at elevations in excess of 4000 m. Here, we demonstrate that alpine bumble-bees possess substantial aerodynamic reserves, and can sustain hovering flight under hypobaria at effective elevations in excess of 9000 m, i.e. higher than Mt. Everest. Modulation of stroke amplitude and not wingbeat frequency is the primary means of compensation for overcoming the aerodynamic challenge. The presence of such excess capacity in a high-altitude bumble-bee is surprising and suggests intermittent behavioural demands for extreme flight performance supplemental to routine foraging.
Keywords: air density, altitude, hovering, hypobaria, insect, kinematics
1. Introduction
Significant reductions in air density and oxygen constrain animal flight at high altitudes [1,2]. Forces produced by flapping wings are directly proportional to air density, so that in the absence of morphological or kinematic compensation, organisms flying at high altitudes will experience drastic reductions in lift forces required to offset body weight [3,4]. Furthermore, flying insects exhibit the highest known mass-specific demand for oxygen [5], and rely in part on passive diffusion driven by differences between internal and atmospheric oxygen partial pressures to sustain high metabolic rates. Therefore, altitudinal reductions in oxygen partial pressure (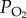 ) may also challenge insect flight. These constraints, along with reduced air temperature, may explain partial or total loss of flight at high elevations in many insect groups (reviewed by [6]). Nonetheless, some insects are capable of active flight at very high altitudes [7].
) may also challenge insect flight. These constraints, along with reduced air temperature, may explain partial or total loss of flight at high elevations in many insect groups (reviewed by [6]). Nonetheless, some insects are capable of active flight at very high altitudes [7].
Although traditionally characterized as bumbling fliers because of their large body size and relatively small wings, bumble-bees (genus Bombus) are regularly found above 4000 m and have been collected above 5600 m [8], where they actively forage among often widely dispersed flowers. Physiological influences on altitudinal limits for bumble-bees and other insects are largely unknown, despite well-characterized kinematic responses to hypodense air [9,10]. Here, we show that alpine bumble-bees are readily capable of flight at simulated altitudes above 8000 m, and that some individuals can hover at barometric pressures that exceed the summit of Mt. Everest (e.g. more than 9000 m). This feat is accomplished, in part, via large increases in the angular extent of wing motion.
2. Material and methods
We captured male Bombus impetuosus (identified by P. Williams, Natural History Museum, London) at flowers near Rilong, Sichuan, People's Republic of China (30.99° N 102.83° E, elevation 3250 m) in August 2005, measured their body mass to the nearest milligram (Acculab PP2060D; Sartorius, Goettingen, Germany), and placed them within a plexiglas flight chamber (30 × 30 × 30 cm) within 10 min of capture. We used male bumble-bees because they were most readily available at the time of the experiments.
After 5 min in the chamber, erratic flights would cease and bees would make repeated, controlled vertical ascents. We scored a bee as capable of hovering flight if it successfully ascended to the top half of the chamber. After observing a successful flight at ambient air pressure (corresponding to approx. 3250 m), we slowly decreased barometric pressure (equivalent to increasing altitude) in the sealed flight chamber using a hand pump, and assessed flight capability at barometric pressures equivalent to approximately 500 m intervals (monitored via a calibrated altimeter in the flight chamber). Maximal flight altitude (figure 1, purple points) was estimated as the altitude halfway between the highest altitude of successful flight (figure 1, blue points) and the lowest altitude of flight failure (clearly indicated by repeated attempts at flight during which the bee was capable of ascending only about one body length above the chamber floor; figure 1, red points). Subsequent to determination of maximal flight altitude, the bee was removed from the chamber and the body and thoracic mass were determined to the nearest milligram [3,11]. Air temperature was monitored to the nearest 1°C via a glass thermometer placed near the top of the chamber and averaged 27 ± 6°C (s.d.) across all flight trials.
Figure 1.
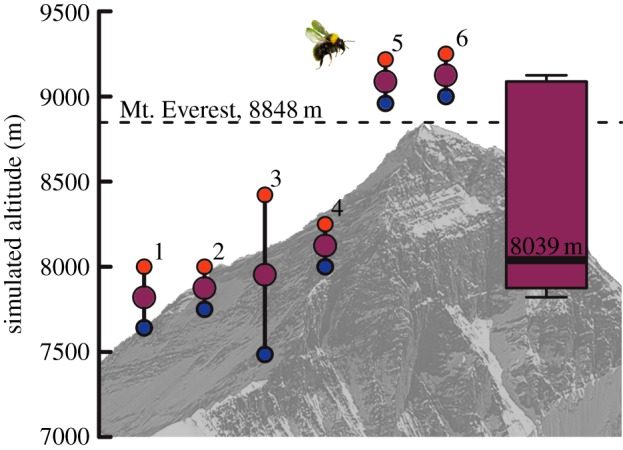
Maximum hybobaric altitudes (purple points and boxplot) estimated as the midpoint between altitudes of the highest successful flights (blue points) and of flight failures (red points) for six bumble-bees captured at 3250 m in western China. Everest image by Pavel Novak and bee image by Sputniktilt; both used and modified under the creative commons licence.
All flight bouts were recorded by a digital video camera (Sony DCR-TRV19) mounted directly above the chamber such that the optical axis was vertical. We analysed flight sequences in which the bee steadily hovered at least two wing lengths from the sides, floor and top of the chamber. We determined wingbeat frequency by acoustic analysis of sound from flight sequences [12]. Flight sequences were de-interlaced (yielding 60 fps) and exported as TIFF images for subsequent digitization (NIH Image, PointPicker package) of the tips of the head and abdomen, and of the wing bases and forewing tips at their maximal extent of angular motion during the up- and downstrokes. Although image capture rate was substantially lower than wingbeat frequency, 30 Hz video de-interlaced to 60 fps gives reliable estimates of stroke amplitude because wingtips are only clearly visible at stroke reversals [11,12]. We determined the minimum, mean and maximum wing positional angles (φmin, φmean and φmax, respectively) as well as the maximum angular extent of wing motion (stroke amplitude, Φ) for left and right forewings from digitized points assuming that the wings moved in a plane parallel to the camera focal plane (i.e. with a stroke plane angle β close to zero, as typically characterizes hovering bumble-bees [9]). We averaged left and right stroke angles to account for small shifts in roll and yaw. Video sequences were analysed in random order without knowledge of bee identity or equivalent altitude. We estimated digitization error by reanalysing eight times four randomly selected video sequences (two each from high and low equivalent altitudes). Digitization errors (mean across video sequences of standard deviations of these eight estimates of kinematic parameters) ranged from 0.6° (φmean) to 2.4° (Φ), indicating that 95% of angular estimates were within 1–5° (i.e. 2 s.d.) of actual values. See the electronic supplementary material for all morphological and kinematic data.
3. Results
We estimated maximal flight altitude and wing kinematics for male bees of similar body mass (109 ± 14 mg; range of 91–129 mg) and comparable relative thoracic size (35 ± 4% of body mass). Changes in body mass during the course of the experiment were small (12 mg or 10%, on average) and were not significant (paired t-test, t2 = −2.55, p = 0.126). On average, bees were capable of hovering at air pressure equivalents exceeding 8000 m (maximum flight altitude median: 8039 m, mean: 8331 m, range: 7820–9125 m; figure 1). All bees successfully hovered at air pressure equivalents exceeding 7400 m, three flew above 8000 m, and two flew at air pressures corresponding to altitudes higher than the peak of Mt. Everest (figure 1). Among individuals, maximum flight altitude was not influenced by body mass (Spearman's ρ = −0.31, p = 0.712) or by air temperature (ρ = 0.03, p = 0.468), but marginally increased with relative thoracic mass (ρ = 0.71, p = 0.051). The two highest fliers (9089 m and 9125 m; bees 5 and 6 in figure 1) also had relatively large thoraces (approx. 36% of body mass) compared with other bees (with values approx. 31%).
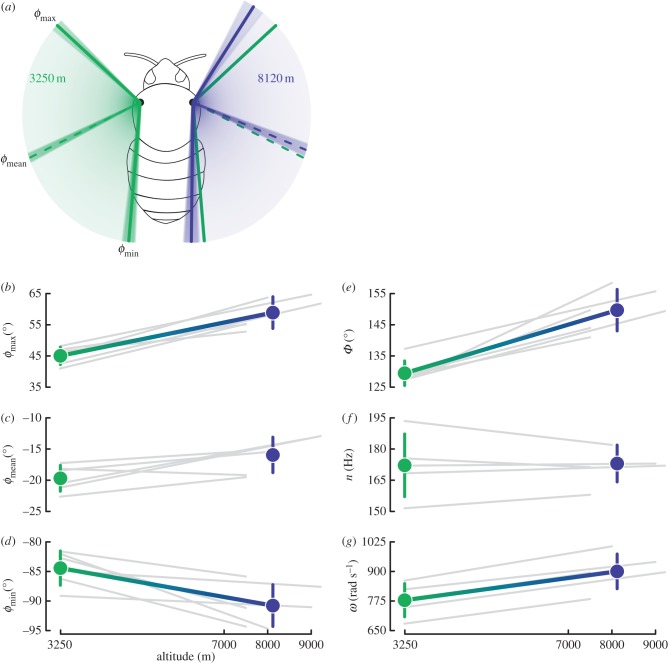
For each bee, we obtained 3.8 ± 1.4 (range 2–6) estimates of wing stroke angles and 4.0 ± 1.6 (range 2–6) estimates of wingbeat frequency from different video sequences at each of two altitudes (i.e. the 3250 m capture altitude and the highest altitude of successful flight; figure 1, blue points). We used mean values across all video sequences for each bee–altitude combination in subsequent analyses. To fly at extreme simulated altitudes, bees increased the angular velocity (ω) of the wings (i.e. twice the product of frequency and amplitude) by increasing stroke amplitude while maintaining a constant wingbeat frequency (table 1 and figure 2e,f,g). The increase in stroke amplitude was driven primarily by a large increase in φmax (approx. 13.9°) and by a smaller shift in φmin (approx. 6.3°), such that bees hovering at high altitudes exhibited values of φmean shifted anteriorly (approx. 3.7°; table 1 and figure 2a–d). The expected pitch up moment induced by this shift, though not measured, was clearly visible as an increase in body angle, particularly at the highest equivalent altitudes.
Table 1.
Differences in flight kinematics of bumble-bees hovering at capture (3250 m) and maximum flight (7500–9200 m) altitudes. Values are means ± 1 s.d. p-values come from paired, two-sided t-tests with significant values in italic based on sequential Bonferroni-adjusted cutoffs with overall α = 0.05.
| 3250 m | maximum altitude | change (95% CI) | t (d.f.) | p-value | |
|---|---|---|---|---|---|
| φmin (°) | −84.4 ± 2.9 | −90.8 ± 3.5 | −6.3 (−2.0, −10.7) | 3.78 (5) | 0.0130a |
| φmax (°) | 45.0 ± 2.8 | 58.9 ± 5.1 | 13.9 (8.1, 19.6) | 6.24 (5) | 0.0015a |
| φmean (°) | −19.7 ± 2.1 | −15.9 ± 2.8 | 3.7 (0.1, 7.4) | 2.66 (5) | 0.0452 |
| Φ (°) | 129.5 ± 3.9 | 149.7 ± 6.6 | 20.2 (13.2, 27.3) | 7.37 (5) | <0.001a |
| n (Hz) | 172.2 ± 15.1 | 171.3 ± 8.6 | −0.9 (−9.8, 7.9) | −0.29 (4) | 0.7878 |
| ω (rad s−1) | 389.2 ± 35.2 | 450.2 ± 36.7 | 62.9 (50.8, 75.0) | 14.39 (4) | <0.001 |
aSignificant at the α = 0.05 level for two-sided paired Wilcoxon signed-rank tests.
Figure 2.
Changes in stroke angles during bumble-bee flight at simulated high altitude. (a) Dorsal view of a hovering bee with means (solid lines) and standard deviations (shading around lines) of wing positions at 3250 m (left wing in green) and 8120 m (the average maximum altitude, right wing in blue). The wing positions for 3250 m are also shown in green on the right wing to facilitate comparison. Maximum, mean and minimum wing positional angles (b,c and d, respectively) and stroke amplitude, wingbeat frequency and angular velocity (e,f and g, respectively), for flight at capture and maximum simulated altitudes. Grey lines indicate data for individual bees and coloured points and lines indicate averages among all bees. Lines are only shown for variables that changed significantly between altitudes (table 1).
We evaluated whether variation in maximum flight altitude among individuals was explained by variation in wingbeat kinematics through use of rank correlations (Spearman's ρ with p-values estimated by permutation tests) between changes in kinematic parameters (using each individual's mean values, visualized as slopes of the grey lines in figure 2) and the maximum flight altitude. Larger increases in φmax and φmean resulted in higher maximum flight altitudes (ρ = 0.88, p = 0.008 and ρ = 0.82, p = 0.026, respectively), whereas changes in the minimum positional angle, stroke amplitude and wingbeat frequency were not significantly correlated with maximum flight altitude (p > 0.05 in each case).
4. Discussion
Maximum flight altitudes of insects are largely unknown other than from data obtained remotely with radar, and by the capture of individuals via aerial sampling [10,13]. These studies suggest that free-flying insects typically ascend no higher than 5000 m; however, these are typically insects migrating on air currents, so they benefit little from ascending beyond a few thousand metres [10]. Many insect taxa have been captured terrestrially at 5000–5200 m in the Himalayas, and several fly species and butterflies have, remarkably, been captured near 6000 m [7]. The possibility of only transient presence, perhaps facilitated by upward ambient winds, cannot be excluded for many of these records; nevertheless, bumble-bees (and probably other insects) forage and even nest (M. Dillon 2007, personal observation) above 4000 m.
In this study, bumble-bees were capable of hovering at air pressures corresponding to altitudes in excess of 9000 m. When challenged in hypodense but normoxic gas mixtures, other bees are capable of hovering at similar altitudinal equivalents (i.e. approx. one-third of sea-level density; see also [4]). Moreover, the interpolative methodology used to approximate these values may underestimate altitudinal limits to flight performance. Under hypobaric conditions, bumble-bees increased stroke amplitude by approximately 20° (figure 2a), corresponding to a 15% increase from the starting value at 3250 m. This effect, when combined with little change in wingbeat frequency (figure 2f), resulted in a 16% increase in angular velocity and an estimated 35% increase in steady-state aerodynamic forces (proportional to the square of wing relative velocity). Air density decreases by about 20% over the equivalent altitudinal range, suggesting that the observed changes in wingbeat kinematics could be sufficient for weight offset at extremely high altitudes. Changes in more subtle features of wing motion (e.g. increased angle of attack, and the timing of wing rotation at the ends of half-strokes) cannot, however, be excluded.
Flight failure in bumble-bees was characterized here as progressive impairment of the aerodynamic capacity to offset body weight. Reduced oxygen partial pressures at higher altitudes may also limit flight performance. However, the maximum stroke amplitudes obtained for bumble-bees obtained here (145–150°) match well with those from studies of maximum flight performance in other bee taxa under both normoxic and hypoxic conditions [3,4,11,12]. As in hummingbirds [14], anatomical limits to wing motions in the stroke plane may ultimately limit maximum hovering performance among the Apidae. Moreover, the highest fliers among all individuals showed the greatest changes in stroke amplitude, driven by the large increases in maximum positional angle (table 1). Wingbeat frequency changes were, by contrast, small.
Because bumble-bees are social insects with high nest fidelity, the presence of foraging individuals in the wild is consistent with residence at elevations close to the point of capture (e.g. ±500 m). The male reproductives studied here may range more widely, although museum records for this species at the Institute of Zoology in Beijing (M. Dillon & R. Dudley 2007, personal observation) suggest an altitudinal range for this species of 1850–4480 m. Over such a range from mid-montane to alpine conditions, the capacity to modify force output may be an essential chronic feature of foraging trips across elevational gradients, but could also reflect the need for short-term burst performance in, for example, mating behaviour [15] or escape from predators. Whether worker bumble-bees (females) are capable of similarly high elevation flights is unknown. Commercially reared Bombus impatiens workers are capable of a 50% increase in vertical force production to lift loads [11], but females have smaller wings relative to body size [2] which could limit flight capacity at extreme elevations [1,2]. The extreme flight performance under hypobaria documented here is unexpected and suggests that routine hovering, while aerodynamically challenging, should not be viewed as an upper bound to aerial performance.
Acknowledgements
We thank G. Cong, Y. Zeng and X. Wang for help in the field and three anonymous reviewers for comments that improved the manuscript.
Funding statement
This work was supported by an NSF Minority Postdoctoral fellowship to M.E.D., and a National Geographic Society CRE grant to M.E.D. and R.D.
References
- 1.Altshuler DL, Dudley R. 2006. The physiology and biomechanics of avian flight at high altitude. Integr. Comp. Biol. 46, 62–71 (doi:10.1093/icb/icj008) [DOI] [PubMed] [Google Scholar]
- 2.Dillon ME, Frazier MR, Dudley R. 2006. Into thin air: physiology and evolution of alpine insects. Integr. Comp. Biol. 46, 49–61 (doi:10.1093/icb/icj007) [DOI] [PubMed] [Google Scholar]
- 3.Roberts SP, Harrison JF, Dudley R. 2004. Allometry of kinematics and energetics in carpenter bees (Xylocopa varipuncta) hovering in variable-density gases. J. Exp. Biol. 207, 993–1004 (doi:10.1242/jeb.00850) [DOI] [PubMed] [Google Scholar]
- 4.Altshuler DL, Dickson WB, Vance JT, Roberts SP, Dickinson MH. 2005. Short-amplitude high-frequency wing strokes determine the aerodynamics of honeybee flight. Proc. Natl Acad. Sci. USA 102, 18 213–18 218 (doi:10.1073/pnas.0506590102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison JF, Roberts SP. 2000. Flight respiration and energetics. Annu. Rev. Physiol. 62, 179–205 (doi:10.1146/annurev.physiol.62.1.179) [DOI] [PubMed] [Google Scholar]
- 6.Hodkinson ID. 2005. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol. Rev. 80, 489–513 (doi:10.1017/S1464793105006767) [DOI] [PubMed] [Google Scholar]
- 7.Mani MS. 1968. Ecology and biogeography of high altitude insects. The Hague, The Netherlands: Dr. W. Junk N. V. Publishers [Google Scholar]
- 8.Williams PH, Ito M, Matsumura T, Kudo I. 2010. The bumblebees of the Nepal Himalaya (Hymenoptera: Apidae). Insecta Matsumurana Ser. Entomol. New Ser. 66, 115–151 [Google Scholar]
- 9.Dudley R, Ellington CP. 1990. Mechanics of forward flight in bumblebees: I. Kinematics and morphology. J. Exp. Biol. 148, 19–52 [Google Scholar]
- 10.Johnson CG. 1969. Migration and dispersal of insects by flight. London, UK: Methuen & Co. Ltd [Google Scholar]
- 11.Buchwald R, Dudley R. 2010. Limits to vertical force and power production in bumblebees (Hymenoptera: Bombus impatiens). J. Exp. Biol. 213, 426–432 (doi:10.1242/jeb.033563) [DOI] [PubMed] [Google Scholar]
- 12.Dillon ME, Dudley R. 2004. Allometry of maximum vertical force production during hovering flight of neotropical orchid bees (Apidae: Euglossini). J. Exp. Biol. 207, 417–425 (doi:10.1242/jeb.00777) [DOI] [PubMed] [Google Scholar]
- 13.Drake VA, Reynolds DR. 2012. Radar entomology: observing insect flight and migration. Boston, MA: CABI [Google Scholar]
- 14.Chai P, Dudley R. 1995. Limits to vertebrate locomotor energetics suggested by hummingbirds hovering in heliox. Nature 377, 722–725 (doi:10.1038/377722a0) [Google Scholar]
- 15.Williams PH. 1991. The bumble bees of the Kashmir Himalaya (Hymenoptera: Apidae, Bombini). Bull. Br. Mus. Nat. Hist. 60, 1–204 [Google Scholar]