Figure 2.
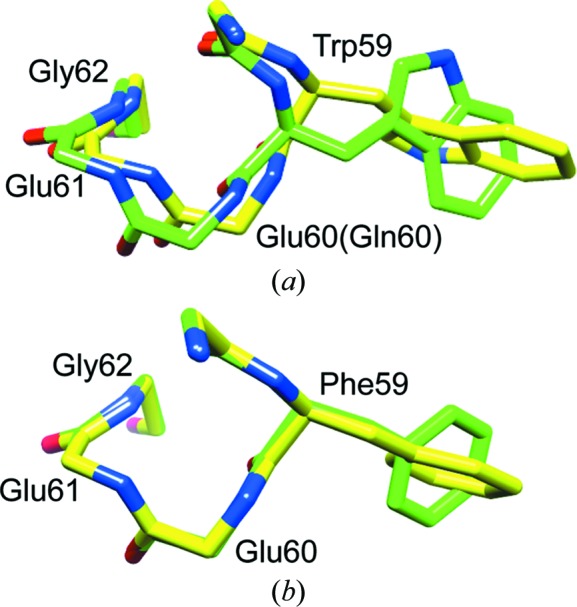
Backbone conformation in the active site of wild-type and E60Q FKBP12 and of the crystallographically non-equivalent monomers of FKBP12.6 in the P3121 crystal form. (a) shows the shift in backbone conformation and the reorientation of the Trp59 side chain that results from the altered hydrogen-bonding interactions of the side chain of residue 60 (Szep et al., 2009 ▶). A similar ∼90° rotation of the side-chain χ2 dihedral angle for Phe59 occurs between the two non-equivalent monomers in the P3121 crystal form of the cysteine-free variant of FKBP12.6, but without any corresponding alteration in the conformation of the backbone (b).
